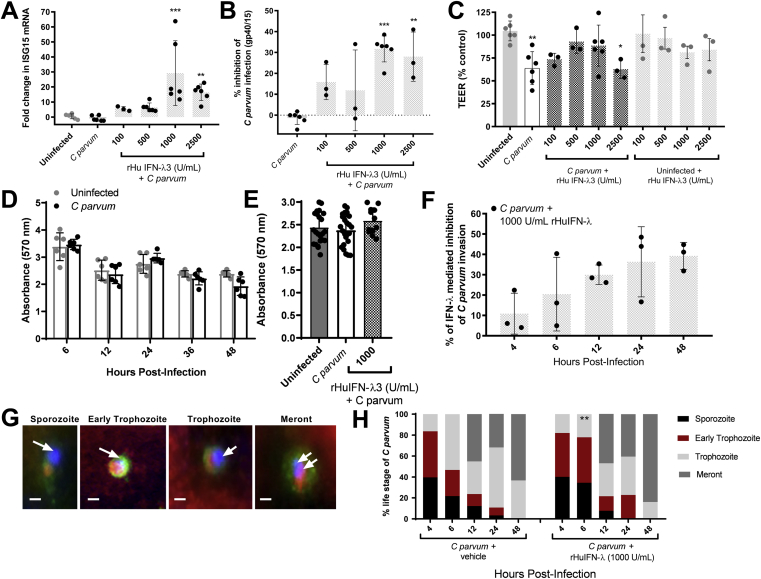
Figure 6.
Priming of IECs with exogenous IFN-λ3 decreases the burden of C parvum infection, abrogates loss of TEER, and enhances resistance to C parvum invasion. Polarized monolayers of IPEC-J2 cells were pretreated with either vehicle, or 100, 500, 1000, or 2500 U/mL of rHuIFN-λ3 for 12 hours before C parvum infection. At the time of peak C parvum infection (12–24 hours after infection) the effect of rHuIFN-λ3 was assessed by qRT-PCR analysis of (A) ISG15 mRNA expression, (B) Cryptosporidiumgp40/15 mRNA content, and by (C) measurement of TEER. The Ct for ISG15 and Cryptosporidiumgp40/15 mRNA content of each monolayer was normalized to expression of the housekeeping gene cyclophilin (ΔCt). For each treatment group, fold-change differences in ISG15 and Cryptosporidiumgp40/15 were expressed as compared with a (A) representative uninfected monolayer or a (B) representative infected monolayer, respectively, using the 2-ΔΔCt method. **P < .01, ***P < .001, 1-way analysis of variance comparison between treatment groups and (A) uninfected or (B) infected monolayers. For TEER (C), individual data points are expressed as a percentage of a representative uninfected monolayer. *P < .05, **P < .01, 1-way analysis of variance comparison between treatment groups and uninfected monolayers. Bars represent means ± SD. (A–D) Results were obtained from 2 independent experiments and combined. (D) Spectrophotometric assay of crystal violet absorbance (570 nm) by IPEC-J2 monolayers after a 48-hour time course of C parvum infection. N = 6 monolayers per treatment and time group. Figure contains results obtained from 1 experiment. (E) Spectrophotometric assay of crystal violet absorbance in individual uninfected control and C parvum–infected monolayers 24 hours after infection and in monolayers primed with 1000 U/mL of rHuIFN-λ3 12 hours before the infection. Figure combines results obtained from 2 independent experiments. Bars represent means ± SD. (F) Intestinal epithelial (IPEC-J2) monolayers were grown on chamber slides and pretreated with either vehicle (n = 15 monolayers) or 1000 U/mL of rHuIFN-λ3 (n = 15 monolayers) for 12 hours before a 48-hour time course of C parvum infection. Three monolayers from each treatment group were examined using fluorescence microscopy at 4, 6, 12, 24, and 48 hours after infection, at which times quantification of invading C parvum parasites was determined by counting the total number of FITC–C parvum and Alexa-555 phalloidin co-labeled parasites within ten 400× fields per monolayer. At each time point, data compare the percentage reduction in C parvum invasion observed in 3 individual rHuIFN-λ3–treated monolayers compared with the average C parvum invasion observed in 3 individual vehicle-treated monolayers. Bars represent means ± SD. Figure contains results obtained from 1 experiment. (G) Representative fluorescence microscopy images of C parvum asexual intracellular life stages. Parasites shown reflect positive labeling with FITC–C parvum (green), DAPI-positive C parvum nuclei (blue and indicated by arrows), and Alexa-555–phalloidin cellular invasion sites (red). Scale bar: 1 μm. (H) The relative percentage of C parvum asexual life stages at each time point in vehicle-treated (left) and rHuIFN-λ3–pretreated infected monolayers. Parasite life stages were counted and classified within five 1000× fields for each monolayer. Data represent an average of n = 3 monolayers per time and treatment group. **P < .01, chi-square comparison of the percentage of trophozoites with vehicle-treated monolayers at the same time point.