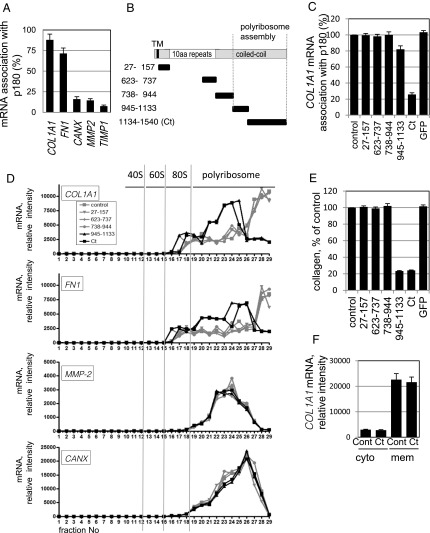
Fig. 1.
The C-terminal coiled–coil domain of p180 associates with target mRNAs and promotes efficient protein synthesis. (A) Ribosome-stripped membrane fractions from ascorbate-treated HEL cells were immunoprecipitated with an anti-p180 antibody. The relative amounts of mRNA were analyzed for COL1A1, FN1, CANX, MMP2, and TIMP1 cDNA recovered in the p180 immunoprecipitates; percentages of the input value are depicted. Data represent means ± SD (n = 4). (B) Structures of full-length p180 and a series of truncated proteins are illustrated. TM, transmembrane domain; white box, a highly basic tandem repeat domain; gray box, a C-terminal acidic coiled–coil domain. Each truncated mutant protein contains the indicated amino acid residues of human p180. (C) The series of green fluorescent protein (GFP)-tagged p180 truncated polypeptides shown in B were expressed in HEL cells. Relative mRNA amounts (vs. mock-transfected cells, set as 100%) recovered in p180 immunoprecipitates of the membrane fractions are depicted. (D) Analyses from sucrose density gradient centrifugation of the membrane fractions are shown. Relative amounts of the indicated mRNAs estimated by qPCR analysis are depicted. Total RNA profiles are shown in SI Appendix, Fig. S1B. (E) Collagen secreted from HEL cells overexpressing a series of polypeptides was quantified by MS analysis. The amount of collagen secreted from mock-transfected cells was set as 100%. (F) After overexpression of Ct as in C, the relative amounts of COL1A1 mRNA in the cytosolic and membrane fractions were compared. Data in C, E, and F represent means ± SD (n = 3).