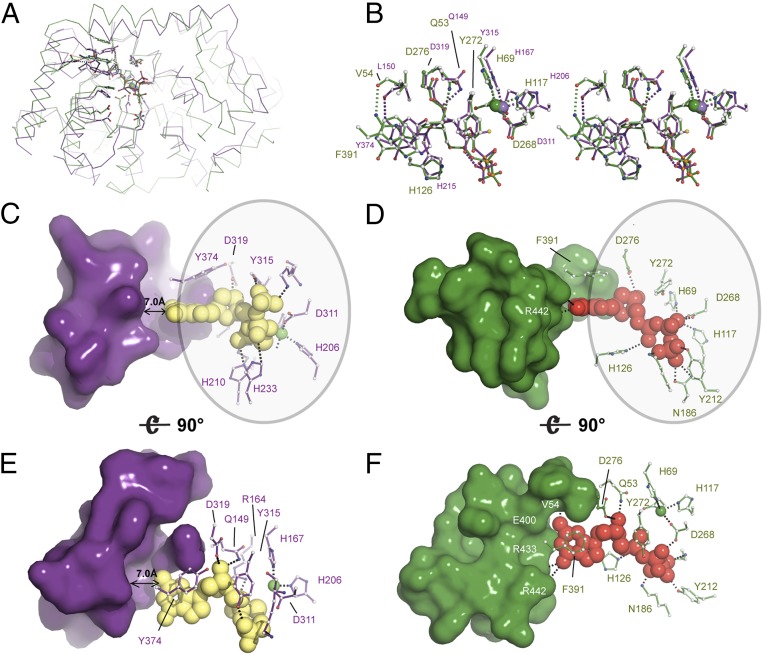
Fig. 5.
Structural and enzymatic insight into the mechanism of Ec-dGTPase activity. (A) Ribbon representation of the overlay between SAMHD1 (PDB ID code 4BZC) (purple) and Ec-dGTPase (green) illustrating fold conservation of the enzymatic cores. (B) Stereo and ball-and-stick representation of the overlay between SAMHD1 and Ec-dGTPase active sites illustrating residue type and geometry conservation. (C and E) Surface and ball-and-stick representation of SAMHD1 active-site residues illustrating that most contacts with the dNTP involve interaction with the ribose, the phosphates, and the purine or pyrimidine ring (circle). No interactions with SAMHD1 residues that could confer specificity are possible since a 7-Å gap separates them from the dNTP. Thus, dNTPases bind shared motifs D and F. A similar set of interactions takes place in dGTPase; however, dGTP selectivity occurs through formation of four hydrogen bonds.