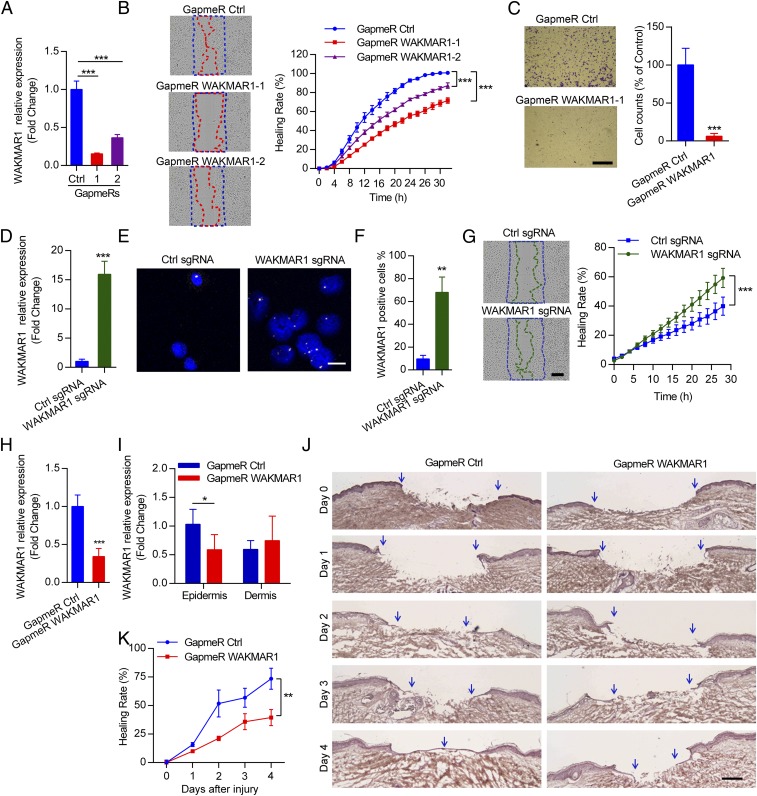
Fig. 3.
WAKMAR1 regulates keratinocyte motility and wound reepithelialization. (A) qPCR analysis of WAKMAR1 in keratinocytes transfected with WAKMAR1-specific GapmeR1, GapmeR2, or control oligos (Ctrl) (n = 3). (B) Scratch wound assay of keratinocytes after WAKMAR1 knockdown (n = 10). (C) Representative photographs of transwell migration assay for keratinocytes with WAKMAR1 knockdown (n = 3). (Scale bar, 1 mm.) The number of cells passing through the membrane was counted. qPCR (D) and ISH (E and F) of WAKMAR1 in keratinocytes transfected with CRISPR/Cas9-SAM plasmids for 48 h are shown. Cell nuclei were costained with DAPI. (Scale bar, 50 μm.) (G) Scratch wound assay of keratinocytes with WAKMAR1 expression activation (n = 10). (Scale bar, 300 μm.) qPCR analyses of WAKMAR1 in full-depth biopsies (H) and in LCM-isolated epidermis and dermis (I) of human ex vivo wounds after topical application of WAKMAR1 GapmeRs for 4 d (n = 6 donors) are shown. (J) Representative photographs of hematoxylin and eosin staining of ex vivo wounds. Blue arrows demarcate the initial wound edges (day 0) and newly formed epidermis (days 1–4). (Scale bar, 200 μm.) (K) Reepithelialization was quantified as healing rate = 100% − percentage of the initial wound size. *P < 0.05; **P < 0.01; ***P < 0.001 by unpaired two-tailed Student’s t test (A, C, D, F, H, and I) or two way-ANOVA (B, G, and K). Data are presented as mean ± SD and are representative of at least two independent experiments.