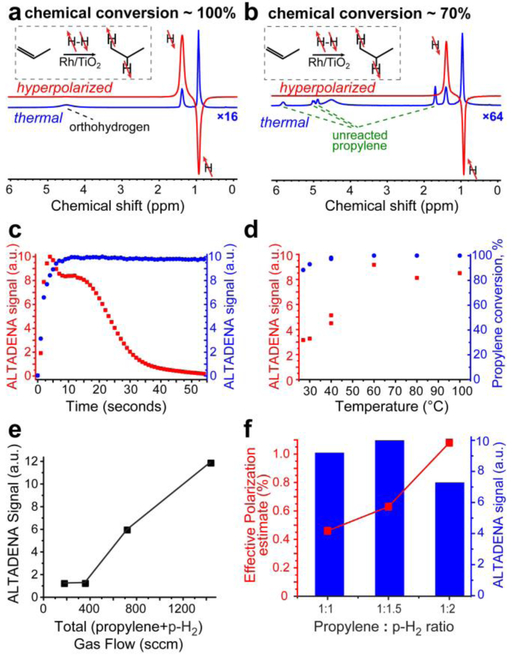
Figure 2.
(a) ALTADENA 1H NMR spectrum of continuously flowing HP propane gas and the corresponding thermal 1H NMR spectrum acquired after interruption of gas flow (the thermal spectrum is multiplied by a factor of 16) under conditions of complete chemical conversion; catalyst mass = 280 mg, t = 130 °C, pressure = 90 psig, gas flow rates: 480 sccm for propylene and 960 sccm for p-H2; signal enhancement ~ 180, P1H ~ 0.58%. (b) ALTADENA 1H NMR spectrum of continuously flowing HP propane gas and the corresponding thermal 1H NMR spectrum acquired after interruption of gas flow (the thermal spectrum is multiplied by a factor of 64) under conditions of partial (~70%) chemical conversion; conditions: catalyst mass = 118 mg, t = 100 °C, pressure = 90 psig, gas flow rates 480 sccm for propylene and 960 sccm for p-H2. The catalyst was extensively treated with cyclopropane before this test (see main text for details); signal enhancement ~ 550, P1H = 1.8%. The insets in displays a) and b) show the reaction scheme of pairwise addition of parahydrogen to propylene to form hyperpolarized propane. (c) Dependence of ALTADENA signal of CH2 group of HP propane obtained in batch mode (red squares) and in continuous flow mode (blue circles) on time on stream. Conditions for batch mode: propylene/p-H2 ratio = 1 : 1.5, catalyst mass = 118 mg, t = 100 °C. Conditions for continuous flow mode: catalyst mass = 118 mg, t = 120 °C, pressure = 90 psig, gas flow rates 480 sccm for propylene and 960 sccm for p-H2. The plots are presented on different scales with the maximum ALTADENA signal in each set of experiments calibrated as 10 a.u. (d) Dependence of ALTADENA signal of CH2 group of HP propane (red squares) and propylene conversion (blue circles) on reaction temperature obtained in batch-mode propane hyperpolarization; conditions: propylene/p-H2 ratio = 1 : 1.5, catalyst mass = 118 mg, average gas flow rate ~4000 sccm. (e) The dependence of PHIP signal of HP propane on the gas flow in continuous-flow operation mode; conditions: propylene/p-H2 ratio = 1 : 2, catalyst mass = 118 mg, t = 100 °C, pressure = 90 psig, chemical conversion was ~100% in all cases. (f) The dependence of HP propane effective polarization estimates on the propane : p-H2 ratio using batch-mode production; conditions: catalyst mass = 118 mg, t = 100 °C. All data is acquired using 9.4 T NMR spectrometer. Note the display f) have dual axes of ALTADENA signal (in arbitrary units, a.u.), which has been employed to compute the effective polarization estimate via signal referencing to the thermally polarized signal.