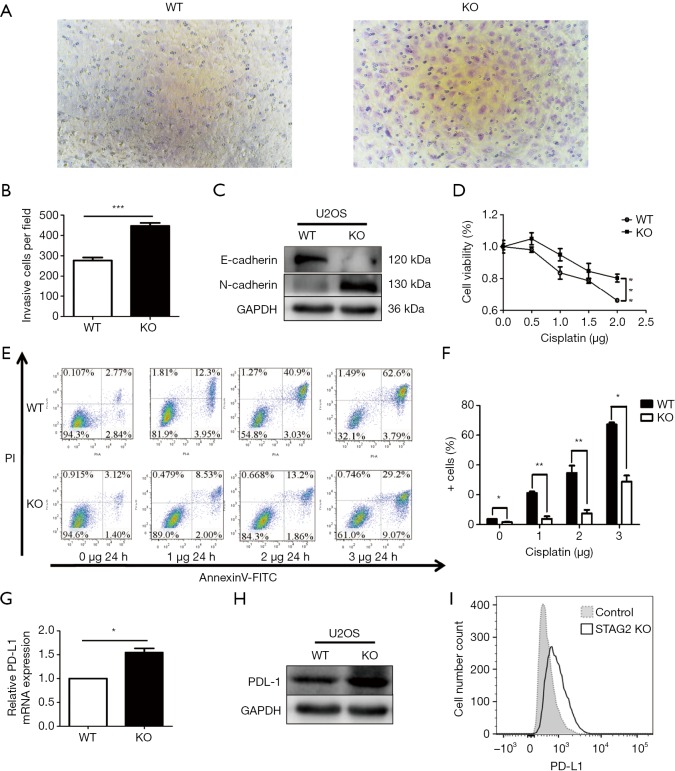
Figure 2.
STAG2 truncating mutation enhanced cell migration ability, Cisplatin-resistance, and PD-L1 expression. (A,B) Cell migration was detected with Transwell chambers (×200). Cells were cultured on the upper chamber in the serum-free media. The lower chamber with the media containing 10% FBS was used as a chemoattractant. The migration cell on the lower chamber was determined under the microscope after 14-hour culture. A t-test was used. ***P<0.001. (C) Western blot assay was used to assess the changes in E-cadherin and N-cadherin protein expression levels and GAPDH as a loading control. (D) Dose-response curves of cisplatin. Cellular viability was measured by the CCK-8 assay after incubating with gradient doses of cisplatin for 24 h. Data from 3 independent experiments are shown as mean ± SD. ***P<0.001 by two-way ANOVA. (E,F) U2OS cells with or without knocked out STAG2 were treated with cisplatin as indicated. The percentage of cell apoptosis was analyzed by flow cytometry. The data represent the mean ± SD of 3 independent experiments *P<0.05, **P<0.01 versus control group. (G) RT-PCR was used to detect the mRNA expression levels of PD-L1. The data represent the mean ± SD of 3 independent experiments *P<0.05 by T-test. (H) Western blot assay was used to assess the changes in PD-L1 protein expression levels and GAPDH as a loading control. (I) PD-L1 expression was determined by FACS-analysis in U2-OS WT and KO cells (open profile, anti-PD-L1 antibody, filled profile, negative control). All data are representative of 3 independent experiments.