Abstract
This study aimed to investigate the treatment effects of combining TAE therapy with LY2109761, a transforming growth factor-beta (TGF-β) receptor I kinase inhibitor, on suppressing tumour growth and metastasis. We simulated the changing tumour microenvironment before and after TAE using both in vitro and in vivo models. In vitro, we evaluated the altered migration and invasion properties of HepG2 cells using migration and invasion assays. In addition, western blot analysis was used to investigate molecular mechanisms underlying the biological activities of LY2109761 in HepG2 cells. In vivo, we combined LY2109761 with TAE together in the VX2 rabbit model to evaluate the therapeutic effects of the combination. In vitro, the Smad pathway were substantially activated by hypoxia, and LY2109761 significantly inhibited the Smad pathway under both normoxic and hypoxic circumstances. Furthermore, LY2109761 inhibited cell proliferation, intravasation and metastasis by downregulating Smad-2 phosphorylation and up-regulating E-cadherin expression in both normoxic and hypoxic conditions. In addition, in animals, LY2109761 improved the therapeutic effect of TAE and inhibited intravasation and metastasis after TAE. Based on the observations herein, we concluded that using LY2109761 and TAE in combination for the treatment of VX2 rabbit liver cancer inhibits tumour growth and metastasis, suggesting that such a combination may provide new a target and strategy for interventional liver cancer therapy.
Keywords: Transarterial embolization (TAE), tumour microenvironment, TGF-β/Smad pathway
Introduction
Hepatocellular carcinoma (HCC) is one of the most common tumours, accounting for an estimated 700000 deaths annually worldwide [1]. HCC has become a major health problem worldwide due to its high mortality, morbidity, and increasing incidence rates [2,3]. TAE, recognized as an effective palliative treatment option for patients with advanced HCC, is now widely performed for the treatment of unresectable liver cancers, classified as the intermediate stage according to the Barcelona Clinic Liver Cancer (BCLC) staging system [4-6]. However, although TACE therapy prolongs the survival of many patients, the fact that conventional TAE therapy performed with elution beads or iodine oil cannot thoroughly eradicate tumour cells should not be ignored. Incomplete necrosis of the tumour could aggravate its hypoxic status, and in many cases, tumour cells can adapt to the highly anaerobic microenvironment resulting from TAE via a negative feedback response and incomplete embolization, which are often correlated with recurrence and metastasis of the tumour [7-10].
Recently, the fundamental roles of the tumour microenvironment in the processes of hepatocarcinogenesis, tumour invasion and metastasis have been increasingly identified [11-13]. Some studies have highlighted the cross-talk between tumour cells and their surrounding microenvironments as well as the fundamental role of the tumour microenvironment in HCC pathogenesis. Cancer-associated fibroblasts (CAFs) are the most prominent cell type within the tumour stroma of many cancers (most notably breast and pancreatic carcinoma) and play a critical role in tumour-stromal interactions [14,15]. HCC cell growth, intravasation and metastatic spread are dependent upon the presence of CAFs, and HCC cells reciprocally stimulate CAF proliferation, suggesting a key role of CAFs in tumour-stromal interactions. Some studies have shown that CAFs are activated by TGF-β and are responsible for the synthesis, deposition and remodelling of excessive extracellular matrix (ECM) proteins, such as various types of collagen [16]. As a vital component of the tumour microenvironment, TGF-β plays a critical role in modulating the biological behaviour of HCC and acts as a double-edged sword role in many cancers, including HCC. For example, TGF-β acts as an onco-suppressor by inhibiting cell proliferation and as a tumour promoter by triggering the epithelial-mesenchymal transition (EMT), which facilitates tumour spreading and metastasis via E-cadherin downregulation. In combination with its receptor (TGF-β receptor 1, TGF-βR1), TGF-β activates the SMAD pathway, which are involved in the biological behaviours underlying HCC cell growth, intravasation and EMT [17-20].
LY2109761, a TGF-β receptor inhibitor, has increasingly been confirmed to suppress the synthesis and release of connective tissue growth factor in the tumour environment, which consequentially reduces the growth, intravasation, and metastatic dissemination of HCC cells via different molecular mechanisms. Previous researches using both a humanized mouse model and human specimens have confirmed that LY2109761 can significantly inhibit the growth and intravasation of tumours [17,21]. In addition, E-cadherin expression was proven to be significantly improved, and EMT was also supressed in these processes.
Considering the high interstitial fluid pressure conditions of solid tumours, such as HCC, especially after the TAE procedure, drugs delivered orally barely reach the lesion comprising residual tumour cells, greatly reducing the therapeutic effects of these drugs. Thus, we evaluated the treatment effects of using TAE therapy and LY2109761 in combination on suppressing tumour growth and metastasis in a rabbit VX2 tumour model. In addition, to better understand the molecular mechanisms underlying the biological activities of LY2109761 after TAE, we simulated an anaerobic environment by downregulating the oxygen concentration to 5% in an in vitro model.
Materials and methods
Reagents
The TGF-β receptor kinase inhibitor LY2109761 was purchased from MedChemexpress (MCE, New Jersey, USA). Cell Counting kit-8 was purchased from Dojindo (Shanghai, China). All antibodies used in cell experiments included a monoclonal antibody against human GAPDH (ANT011, Antgene, Wuhan, China), a monoclonal blocking antibody against human HIF-1α (GTX127309, GeneTex, Southern California, USA), anti-human E-cadherin antibody (20874-1-AP, Proteintech Group Inc., Chicago, USA), anti-human TGF-β antibody (ab27969, Abcam, Cambridge, England), anti-human phospho-Smad2 antibody (#3108, Cell Signalling Technology Inc., Massachusetts, USA), anti-human total Smad2 antibody (GTX111075, GeneTex, Southern California, USA), and the horseradish peroxidase-labeled secondary antibodies (ANT019 and ANT020, Antgene, Wuhan, China).
Cell culture
Human HCC cells of the HepG2 line were obtained and then cultured in high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM, Hyclone, Utah, USA) supplemented with 10% foetal bovine serum (Gibco, Thermo Fisher Scientific, Inc., Massachusetts, USA), 100 mg/ml penicillin, and 100 mg/ml streptomycin. The cells were incubated at 37°C in a humidified atmosphere at 21% O2 (normal oxygen conditions) or 5% O2 (hypoxia).
Immunofluorescence analysis
The TGF-β and HIF-α expression in HepG2 cells under normal oxygen or hypoxic conditions were observed by immunofluorescence as described previously [22]. Cells were fixed in 4% paraformaldehyde solution, followed by washing with PBS three times. 10% goat serum was added to block nonspecific binding of antibody. The cells were stained with the following conjugated antibodies: rabbit anti-HIF-α antibody, Alexa Fluor 488-conjugated donkey anti-rabbit IgG secondary antibody (ANT024, Antgene, Wuhan, China), mouse anti-TGF-β antibody, FITC-conjugated goat anti-mouse IgG secondary antibody (AS1112, Aspen, Wuhan, China). The nuclei were then stained with 4,6-diamidino-2-phenylindole (DAPI). After seal with antifade mounting medium, cells were visualized under a fluorescence microscope (Olympus, Japan).
Cytotoxicity assays
The cytotoxicities of LY2109761 on HepG2 cells under normal oxygen and hypoxic conditions were determined by the Cell Counting Kit-8 assay. HepG2 HCC cells were plated in humidified atmospheres of 21% O2 (37°C) or 5% O2 (37°C) and cultured for 24 hours. In addition, the cells were supplemented with LY2109761 at 0.001, 0.01, 0.1, 1, 10 and 20 µM as well as with the CCK8 solution (10 µL). Each experimental condition was reproduced in 8 wells, and each experiment was repeated 3 times. To confirm the cytotoxic data, cells were incubated under the described conditions for 4 hours, and absorbances were measured at 450 nm using a microplate reader.
Wound healing assay
HepG2 cells were seeded in 6-well plates and grown to confluency in complete medium under normal oxygen and hypoxic conditions. The monolayers were pre-treated with LY2109761 at 0, 0.01 µM and 1 µM for 24 hrs before being scratched with a plastic tip. Wound closures were then monitored by microscopy at 100× magnification.
Migration/invasion assay
To evaluate alterations in tumour cell migration and invasion properties, cell migration assays were performed. Cells were serum-starved overnight and then cultured in transwell chamber in the absence or presence of LY2109761 at concentrations ranging from 0.001 µM to 10 µM from 0 to 48 hours. The cells that passed through polycarbonate membrane were stained with crystal violet and observed with a light microscope (Olympus, Japan) at 100× magnification.
Western blot analysis
The cells were resuspended in cell lysis buffer (pH 8.0) comprising 50 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 1% NP40, 0.05% PMSF, 2 mg/ml aprotinin, and 2 mg/ml leupeptin. Approximately 20 ug of protein/well was loaded onto the gels. The targeted protein levels were determined using western blot analysis as described previously.
Animals and treatments
New Zealand white rabbits weighing 2.5-3.0 kg were purchased from the Experimental Animal Center of the Huazhong University of Science and Technology for experimental use in these studies. All the experimental protocols were approved by the Animal Experiment Committee of the Institute for Huazhong University of Science and Technology.
The rabbit VX2 hepatocellular carcinoma model was established as follows: (1) VX2 tumour cells were implanted inside the hind leg muscles of the rabbits, yielding substantial palpable masses 2 weeks after implantation, thus creating the tumour-bearing rabbits. (2) One tumour-bearing rabbit was sacrificed, and the auxetic VX2 tumour was dissected and separated into 1×1×1 mm3 pieces under sterile conditions and then stored in physiological saline. (3) The operation was performed via abdominal median incision, subsequently inoculating the VX2 tumour into the left lobe of the liver in the candidate rabbits. The puncture was covered by gelatin, and the left liver lobe was then placed back into the abdomen. (4) All of the rabbits were administered penicillin intramuscularly for three days after implantation of the VX2 tumour. The tumours were allowed to grow in the rabbits’ livers for 14 days.
TAE procedure
TAE treatment was performed on rabbits implanted with a VX2 liver tumour one day after perfusion computed tomography (CT) scanning. In total, 30 rabbits were randomly divided into three groups (each group included 10 rabbits): Group I-LY+Gel (1 mL of ultra-liquid iodized oil containing the LY2109761-DMSO suspension at a concentration of 10 mg/kg and gelatin sponge, TAE), Group I+Gel (1 mL of ultra-liquid iodized oil supplemented with gelatin sponge, TAE), and Group NS (1 mL of normal saline solution).
All rabbits were treated under general anaesthesia, and the arteria cruralis of each rabbit was bluntly dissected. Subsequently, a catheter guide wire was moved to the hepatic tumour feeding artery from the arteria cruralis under the guidance of digital subtraction angiography (DSA). Then, the various drugs of the different groups were separately injected into the feeding arteries of the rabbits. Finally, the arteria cruralis of each rabbit was sutured, and all rabbits were intramuscularly administered penicillin for three days.
Measuring tumour size
Perfusion CT was used to estimate the tumour sizes in the experimental rabbits 14 days after implantation of the VX2 tumour tissues. All the rabbits were anaesthetized by chloral hydrate (10%). After scanning, all the acquired images were processed by the Syngo Fastview image processing system, and the sizes, locations, and shapes of the implanted tumours as well as whether necrosis and intrahepatic metastasis were present were analysed by two senior doctors of the radiology department. The maximum diameters (A1) and transverse diameters (B1) of the tumours were separately recorded, and the tumour sizes (V1) were calculated as “V1=A1×B1 2/2”.
All the experimental rabbits subjected to TAE treatment were scanned by perfusion CT 10 days after the operation. The sizes, locations and shapes of the tumours as well as whether necrosis and intrahepatic metastasis were present in the rabbits were reanalysed. The maximum diameters (A2) and transverse diameters (B2) of the tumours were separately recorded, and the tumour sizes (V2) were calculated as “V2=A2×B2 2/2”. The tumour growth rates (TGR) were also calculated as “TGR (%) =V2/V1”.
Tissue analysis
All animals were euthanized with an overdose of chloral hydrate (10%) (Laboratory Animal Center, HuaZhong University of Science and Technology, Wuhan, China). The tumour and surrounding liver tissues were removed. Every tissue sample was bisected, with each half including the tumour and surrounding liver tissues. All the liver tissues were embedded in Optimal Cutting Temperature compound (Sakura, Tokyo, Japan) and cut into 10-μm-thick frozen slices at the greatest tumour diameter for immunofluorescence analysis as described previously [22]. The primary antibodies used for tissue immunofluorescence included rabbit anti-HIF-α antibody (GTX127309, GeneTex, Southern California, USA), mouse anti-TGF-β antibody (ab27969, Abcam, Cambridge, England), rabbit anti-Ki67 (ab92742, Abcam, Cambridge, England), mouse anti-CD31 (M0823, Dako, Denmark), and rabbit anti-E-cadherin antibody (20874-1-AP, Proteintech Group Inc., Chicago, USA). The corresponding secondary antibodies included Alexa Fluor 488-conjugated donkey anti-rabbit IgG antibody (ANT024, Antgene, Wuhan, China), FITC-conjugated goat anti-mouse IgG antibody (AS1112, Aspen, Wuhan, China), and Alexa Fluor 594-conjugated donkey anti-rabbit IgG antibody (ANT027, Antgene, Wuhan, China). Briefly, after fixation in 4% paraformaldehyde and blocking with 10% goat serum, the sections were stained with primary antibodies overnight at 4°C. These antibodies were detected with a fluorescence-labelled secondary antibody, and the nuclei were then stained with DAPI. Finally, the sections were sealed with antifade mounting medium and visualized under a fluorescence microscope (Olympus, Japan) or an Olympus FV1000 (Japan) confocal laser scanning microscope.
Statistical analysis
All data are presented as the mean ± SD. Measurement data were analysed statistically by one-way ANOVA and double-factor variance analysis. P<0.05 was considered statistically significant, and all line figures were drawn using GraphPad Prism 6.0 (GraphPad Software, California, USA).
Results
Conventional TAE killed most tumour cells but did not thoroughly induce necrosis of the tumour lesion
As mentioned above, TAE is recognized as one of the most important therapeutic methods for treating HCC, as it has prolonged the survival of HCC patients in recent years; however, the outcomes of TAE treatment remain modest [23,24]. While TAE can kill most tumour cells, it cannot clinically induce thorough necrosis of the tumour lesion due to some unavoidable reasons. In this study, we also evaluated and compared the necrosis conditions of the I+Gel/microsphere and NS groups. cTAE could kill most tumour cells but could not sufficiently induce thorough necrosis of the tumour lesion, as residual tumour cells remained (Figure 1). In addition, such conditions induced a hypoxic environment, which consequentially stimulated the growth and metastasis of the remaining tumour cells.
Figure 1.
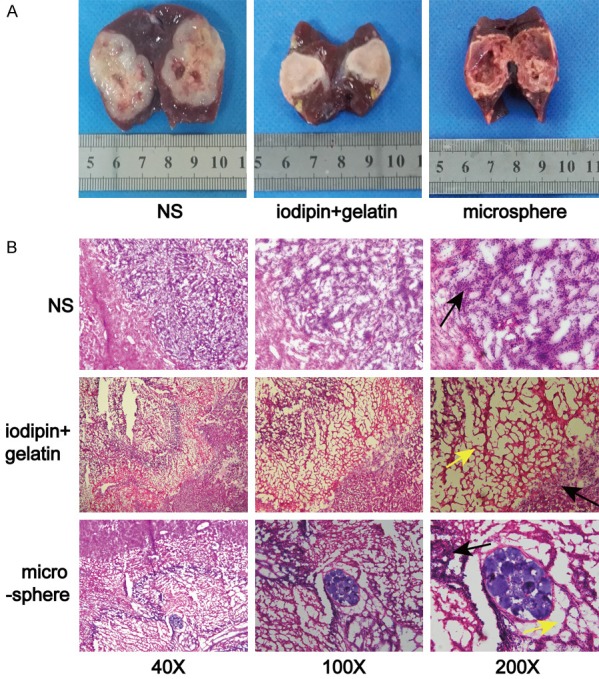
TAE can kill most tumour cells, but residual tumour cells remain. A. Tumour coagulated necrosis can be induced by lipiodol in combination with a gelatin sponge or microsphere embolization. B. Large necrotic tumour areas (indicated by the yellow arrows) were observed after embolization, but residual tumour cells remained (black arrows).
TGF-β and HIF-α overexpression was observed in the tumour microenvironment after TAE or HepG2 cells under hypoxic conditions
While embolization with iodized oil can be used to treat liver cancer by blocking the blood supply to tumour and stroma cells, iodized oil can also function as a cell killer due to its inherit nature. However, in practical clinical work, conventional TAE cannot induce thorough tumour cells necrosis. As described in previous studies, the expression of HIF-1α, a protein induced by hypoxia, was significantly improved after TACE via related pathways [25]. In addition, TGF-β is considered a HCC hallmark because its overexpression is potentially tightly correlated with tumour progression and survival. Hence, in this study, we attempted to evaluate the expression of the two specified factors both in vitro and in vivo. In the vitro model, we simulated the hypoxic condition after TAE by altering the oxygen concentration to 5%, which increased the protein expression levels of Hif-1α and TGF-β in HepG2 cells (Figure 2A). In addition, in the vivo animal model, immunofluorescence analysis showed that the HIF-1α and TGF-β expression levels were increased after TAE in the VX2 hepatocellular carcinoma model compared with those in the placebo group (NS group, Figure 2B).
Figure 2.
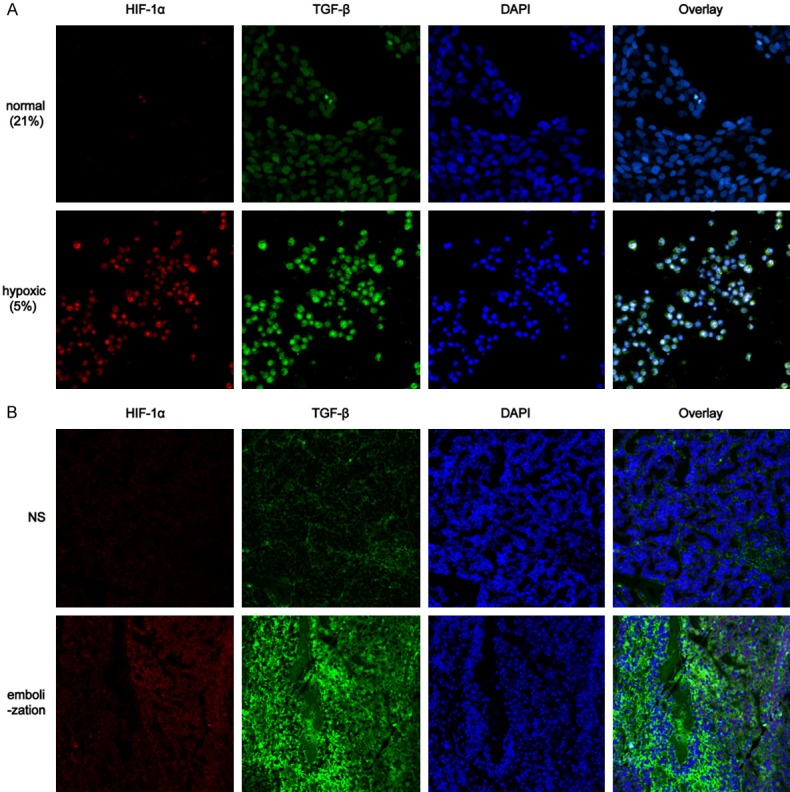
Hypoxia increased the TGF-β and HIF-α expression in vitro and in vivo. A. Hypoxia increased the protein expression levels of HIF-1α and TGF-β and in HepG2 cells compared with those in the normal oxygen group. B. The expression levels of HIF-1α and TGF-β were increased after TAE in the VX2 hepatocellular carcinoma model compared with those in the NS group.
LY2109761 dose-dependently inhibited cell proliferation
In this study, the cytotoxicity of LY2109761 was determined by the CCK-8 assay and trypan blue staining. HepG2 cells were treated with LY2109761 at different concentrations, followed by a 4 h incubation in CCK8 solution under different oxygen concentrations (21% and 5%). The toxicity of LY2109761 was dose-dependent (Figure 3A), ranging from 0.001 to 20 µM; LY2109761 was not cytotoxic from 0.001 to 1 µM but was cytotoxic at 10 and 20 µM. Similar results were obtained with trypan blue staining (Figure 3B).
Figure 3.
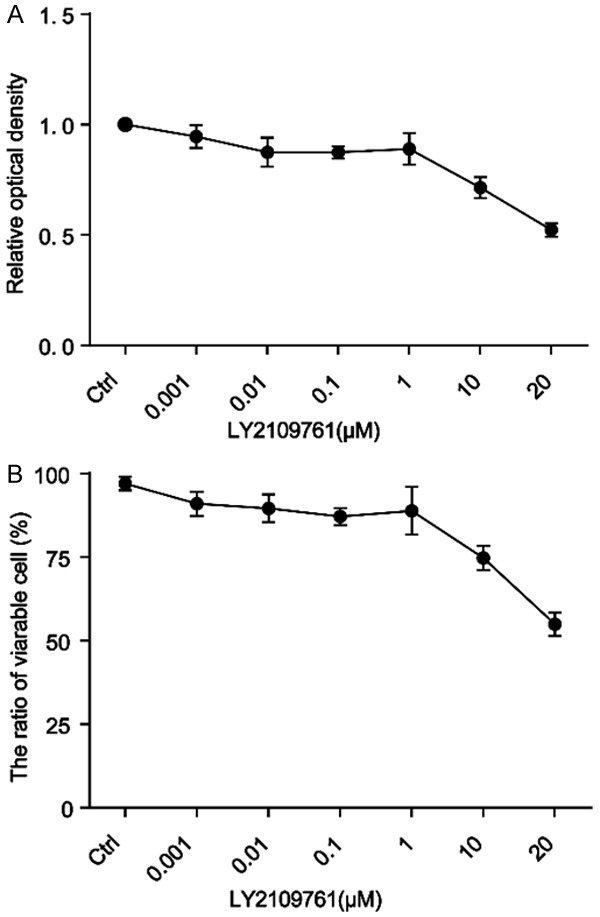
LY2109761 toxicity is dose-dependent. A. LY2109761 toxicity appeared at the 10 µM concentration, as measured by the CCK-8 assay. B. Similar results were obtained with trypan blue staining.
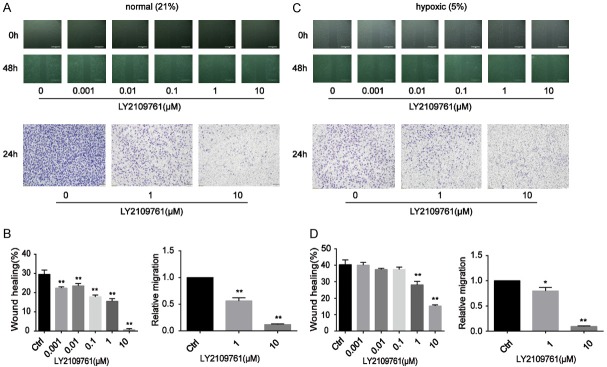
LY2109761 suppressed the migration and invasion of HCC cells in both normal oxygen and hypoxic conditions
In the past decade, TGF-β1 has been recognized as a key driver of liver fibrosis, resulting in a higher risk of HCC development [21,26,27]. In addition, as previously mentioned, TGF-β1 may helped trigger EMT during the tumour growth process, which facilitates tumour spreading and metastasis via downregulating E-cadherin. Some studies have confirmed that the small molecule kinase inhibitor galunisertib selectively blocks TGF-βR1, thereby inhibiting induction of the canonical Smad-2 signalling pathway [28]. Smad-2 inhibition increases the E-cadherin level, reducing the migratory and invasive capabilities of HCC.
Hence, to evaluate alterations in tumour cell migration and invasion properties and to evaluate the inhibitory effect of LY2109761 on the migratory and invasive properties of HCC cells, wound healing and transwell-based migration assays were performed herein in a vitro model under 5% O2 and 21% O2 conditions. As anticipated, LY2109761 significantly suppressed the migration and invasion of tumour cells (Figure 4). It inhibited the migration of HepG2 cells at a very low dose (0.001 µM). Consistent with the wound healing assay result, the invasive property of HepG2 cells was greatly suppressed by LY2109761 at a dose of 1 µM under normal culture conditions (Figure 4A, 4B). LY2109761 also suppressed the migration and invasion of tumour cells under hypoxia (Figure 4C, 4D).
Figure 4.
LY2109761 significantly suppressed the migration and invasion of tumour cells. A, B. LY2109761 inhibited the migration of HepG2 cells at a very low dose (0.001 µM). Consistent with the wound healing assay result, the invasive property of HepG2 cells was greatly suppressed by LY2109761 at a dose of 1 µM under normal culture conditions. C, D. LY2109761 also suppressed the migration and invasion of tumour cells in the hypoxic state. *P<0.05, **P<0.01 versus the control.
Hypoxia substantially activated the Smad pathway, while LY2109761 significantly inhibited this pathway under normoxic and hypoxic conditions
A growing body of evidence highlights that the TGF-β/Smad pathway is tightly associated with the proliferation of tumour cells. Previously studies have proven that the small molecule kinase inhibitor LY2109761 selectively blocks TGF-βR1, thereby inhibiting induction of the canonical Smad-2 signalling pathway. Smad-2 inhibition increases the E-cadherin level, reducing the migratory and invasive capabilities of HCC. Therefore, in this study, we investigated the expression levels of phosphorylated Smad-2 and E-cadherin as well as HIF-1α expression via Western blot analysis.
LY2109761 substantially downregulated pSmad expression in both normoxic and hypoxic conditions (Figure 5A), illustrating that LY2109761 significantly inhibits the pSmad pathway. We also observed that hypoxia inhibited the protein expression of E-cadherin in HepG2 cells, while LY2109761 enhanced E-cadherin expression under normoxic and hypoxic conditions (Figure 5B).
Figure 5.
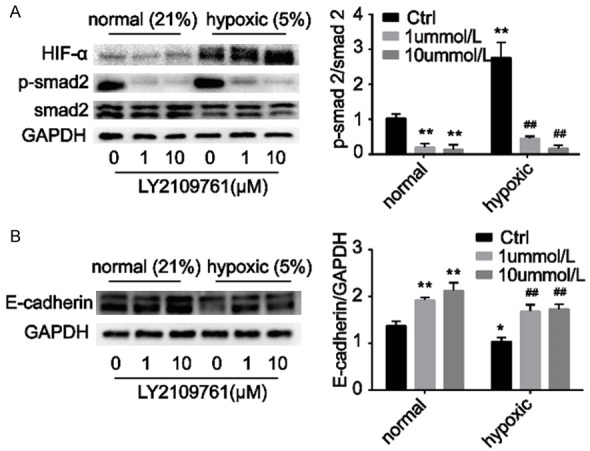
Hypoxia substantially activated the Smad pathway. A. LY2109761 significantly inhibited the Smad pathway under normoxic and hypoxic conditions. B. Hypoxia inhibited the protein expression of E-cadherin in HepG2 cells, while LY2109761 enhanced E-cadherin expression under normoxic and hypoxic conditions. *P<0.05, **P<0.01 versus the control (in normal groups), ##P<0.01 versus the control (in hypoxic groups).
LY2109761 improved the therapeutic effect of TAE and inhibited intravasation and metastasis after TAE in a VX2 hepatocellular carcinoma model
As previously described, LY2109761 inhibits HepG2 cell proliferation in a dose-dependent manner. Thus, we further evaluated the therapeutic effect of LY2109761 in combination with TAE in a VX2 rabbit model. Analysis of the tumour growth rates of the three groups revealed that the growth rate of Group I-LY+Gel was significantly decreased compared with that of the other groups (P<0.05, Figure 6). A histogram (Figure 6C) shows that compared with simple lipiodol embolization, combining TAE with LY2109761 for treatment of the rabbit VX2 liver cancer model better inhibited tumour growth after TAE. Furthermore, we also evaluated intrahepatic metastasis in liver specimens acquired 10 days after the TAE procedure. While the tumours developed coagulation necrosis after embolization, intrahepatic metastases were still visible (Figure 6B). In contrast with the number of intrahepatic metastases in the I+Gel group, that in Group I-LY+Gel was markedly decreased.
Figure 6.
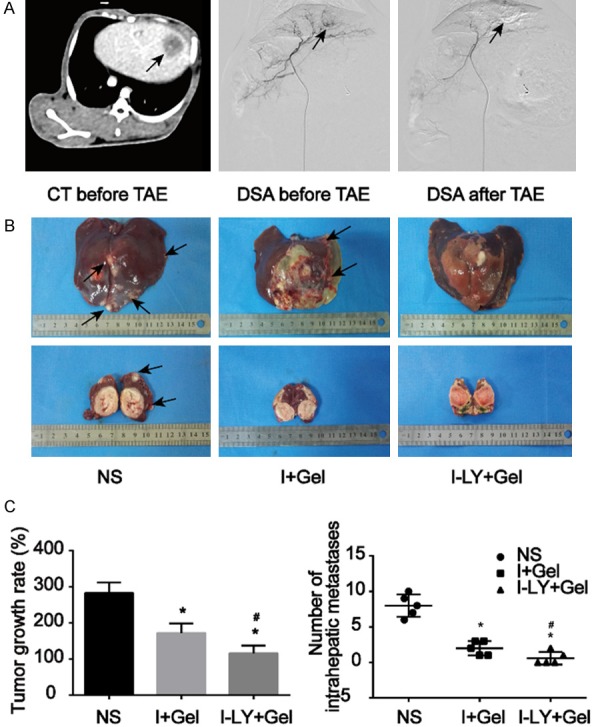
TAE combined with LY2109761 can inhibit tumour growth and metastasis in the rabbit VX2 liver cancer model. A. CT enhancement scan and DSA aimed at liver cancer were performed on rabbits before and after intervention embolization treatment. We observed round tumours in the liver (black arrows), and the blood-supply vessels in the tumours were completely blocked after treatment. B, C. Gross liver specimens were taken ten days after embolization. The number of intrahepatic metastases (black arrow) in the I-LY+Gel group was markedly decreased in contrast with that in the NS/I+Gel group, and the growth rate of Group I-LY+Gel was significantly decreased compared with that of the other groups. *P<0.05 versus the NS group, #P<0.05 versus the I+Gel group.
Expression levels of related proteins involved in HCC growth and metastasis
We further evaluated the expression levels of nuclear antigen Ki67, CD31 and E-cadherin, which are proven to be involved in tumour growth and metastasis, via immunofluorescence staining. The Ki-67 protein (also known as MKI67) is a cellular marker for proliferation and is strictly associated with cell proliferation. Immunofluorescence analysis showed that Ki67 expression in the surviving tumour was increased after TAE treatment, and TAE in combination with LY2109761 inhibited Ki67 expression in the remaining tumour cells (Figure 7A). Similar findings were also showed with CD31, which is known to be tightly correlated with tumour angiogenesis. Staining for CD31 in the I-LY+Gel group was much lower than that in the I+Gel group (Figure 7B), suggesting that TAE combined with LY2109761 inhibits tumour angiogenesis. Moreover, consistent with our results in vitro, E-cadherin expression in the surviving tumour was decreased after TAE treatment, and TAE combined with LY2109761 increased E-cadherin expression on the remaining tumour cells (Figure 7C).
Figure 7.
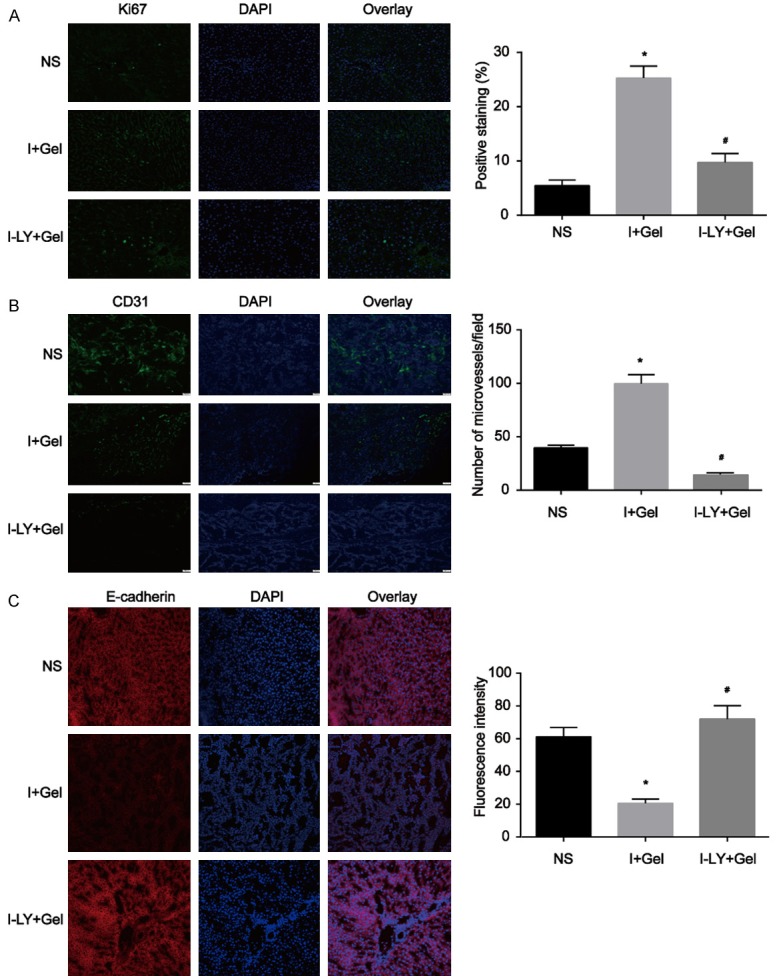
Immunofluorescence analysis of the expression of proteins related to tumour growth and metastasis in the VX2 rabbit model. A. Ki67 expression in the surviving tumour was increased after TAE treatment, and TAE combined with LY2109761 inhibited Ki67 expression in the remaining tumour cells. B. Microvessel generation (CD31 staining) in the residual tumour was increased after TAE treatment, while TAE combined with LY2109761 inhibited microangiogenesis in the residual tumour. C. E-cadherin expression in the surviving tumour was decreased after TAE treatment, and TAE combined with LY2109761 increased the E-cadherin expression on the remaining tumour cells. *P<0.05 versus the NS group, #P<0.05 versus the I+Gel group.
Discussion
Due to the hidden nature of HCC onset, most patients are already in advanced stages of the disease at the time of diagnosis and are thus not candidates for curative treatments, such as surgical resection and liver transplantation [29-31]. Therefore, using different treatment strategies in combination is very important for the treatment of liver cancer. Over the past decade, as our knowledge of tumour pathogenesis has improved, some molecularly targeted agents have begun to emerge. Moreover, in the new era of the war against cancer, molecular targeted therapy based on molecularly targeted agents (MTAs) has gradually became an indispensable component of the treatment regimen for patients with HCC [8]. Sorafenib, which has been reported to prolong the overall survival of HCC patients, is now recommended as the first-line therapy for systemic HCC treatment [32,33]. However, low efficacy and drug resistance hamper the clinical application of MTAs, especially during the treatment of HCC, and new therapeutic methods and targets are thus urgently needed [34]. The intervention and monitoring of key pathways that influence the biological behaviour of HCC is a key starting point [34].
In this study, we simulated a changing tumour microenvironment before and after TAE both in vitro and in vivo. We blocked the blood supply to the tumour via embolization, and injected LY2109761 directly into the lesion. Using animal experiments, we confirmed that the TGF-β receptor inhibitor LY2109761 inhibits tumour growth under hypoxic circumstances, which was consistent with our in vitro results. Furthermore, subsequent experiments on related proteins and factors confirmed that LY2109761 may inhibit liver cancer cell growth, vascular invasion and dissemination spreading by strongly inhibiting the Smad-2 pathway. In addition, via such mechanisms, E-cadherin expression was up-regulated on the hepatocellular carcinoma cell membrane, which further regulated cell adhesion and reduced the migration of hepatoma cells.
TGF-β is a secreted polypeptide shown to have biological activity in tumour cells and induce the oncogenic transformation of noncancerous cells in culture [35]. The TGF-β signal is dysregulated in different types of cancers and thus affects the overall progression to malignancy. For this reason, TGF-β has been considered a valuable target in oncology. A careful clinical study testing doses of the improved TGF-β receptor inhibitor in glioma patients revealed strong beneficial effects and a complete lack of cardiotoxic side effects, advancing the promise of anti-TGF-β therapy [36]. Nonetheless, increased ECM deposition is a characteristic observed in many solid tumours, such as HCC, that also contributes to increased tumour interstitial fluid pressure, blocking the perfusion of anticancer therapies to tumour cells and generally contributing to chemoresistance [37-39].
TAE/TACE is considered an important interventional therapeutic approach to treating HCC patients, as it has several advantages (e.g., a minimally invasive procedure rendering a short recovery time for patients, a tumour-targeted procedure inducing fewer side effects) [9]. However, in many cases, tumour cells adapt to the highly anaerobic microenvironment resulting from TAE/TACE via a negative feedback response. Although interventional embolization in the treatment of liver cancer has played an increasingly important role over the past 30 years [40,41], its overall effects on liver cancer and long-term efficacy has been unsatisfactory, possibly due to changes occurring in the tumour microenvironment after embolization that contribute to the recurrence and metastasis of the residual tumour. Based on domestic and international disciplines and the latest basic research hot spots [42], we considered further studying the impacts of interventional embolization on the liver cancer microenvironment and the corresponding intervention strategies to combat changes in the tumour microenvironment after embolization to be necessary. Until now, no systematic studies on changes in the tumour microenvironment and targeted therapy in HCC patients after interventional embolization have been performed, and no reports on using the TGF-βR inhibitor LY2109761 in interventional HCC therapy exist. Therefore, we propose a new concept of interventional embolization combined with LY2109761 targeted therapy on the liver cancer microenvironment and preliminary exploration in an animal liver cancer model. LY2109761 was shown to exhibit a sufficient adjuvant effect in the treatment of liver cancer embolization.
Our data indicate that it is activation of TGF-β/Smad-2 pathway by an autocrine or paracrine pathway and inhibition of E-cadherin expression in tumour cells under hypoxic condition that leads to tumour proliferation and migration. Although the inhibitory effect of LY2109761 is weakened by the hypoxic environment, LY2109761 can still achieve a good targeted inhibition effect after increasing the concentration. Based on in vitro experiments, we did not use oral or intravenous administration in liver cancer model animals. We directly administered drugs via the tumour supplying artery through intervention, and the local drug concentration in the tumour was greatly increased by precise super-selective administration. At the same time, the subsequent TAE treatment can reduce the drug loss caused by blood flow scouring. TAE treatment can kill tumours in a large amount but the residual hypoxic tumour cells will rapidly proliferate and metastasize. LY2109761 at a higher concentration can effectively inhibit the proliferation and metastasis of hypoxic tumours. Based on the above studies, LY2109761 infusion via tumour-fed artery combined with TAE treatment can play a good synergistic effect.
In summary, LY2109761 can effectively block the TGF-β receptor pathway and up-regulate E-cadherin expression on tumour cell membranes under hypoxic conditions, which inhibits the growth, invasion and migration of the residual tumour after embolization in an anoxic microenvironment. Besides, these findings make combing TAE and LY2109761 a promising potential therapy for interventional embolization. Finally, our efforts have deepened our understanding of interventional liver cancer treatment in the tumour microenvironment field and hopefully provide a new target and strategy for interventional liver cancer therapy.
Acknowledgements
This work was financially supported by grants from the National Natural Science Foundation of China (No. 81571782, No. 81371662 and No. 81470483). This manuscript has been proofread and edited by a professional English editing company, American Journal Experts. We are grateful to the Animal Center, the Central Laboratory Department of Wuhan Union Hospital, Tongji Medical College of Huazhong University of Science and Technology. Songlin Song, Xiaojun He and Zhuanglin Zeng are contributed equally to this work.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Breous E, Thimme R. Potential of immunotherapy for hepatocellular carcinoma. J Hepatol. 2011;54:830–834. doi: 10.1016/j.jhep.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48(Suppl 1):S20–37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet. 2009;373:614–616. doi: 10.1016/S0140-6736(09)60381-0. [DOI] [PubMed] [Google Scholar]
- 7.Lewandowski RJ, Mulcahy MF, Kulik LM, Riaz A, Ryu RK, Baker TB, Ibrahim SM, Abecassis MI, Miller FH, Sato KT, Senthilnathan S, Resnick SA, Wang E, Gupta R, Chen R, Newman SB, Chrisman HB, Nemcek AA Jr, Vogelzang RL, Omary RA, Benson AB 3rd, Salem R. Chemoembolization for hepatocellular carcinoma: comprehensive imaging and survival analysis in a 172-patient cohort. Radiology. 2010;255:955–965. doi: 10.1148/radiol.10091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rammohan A, Sathyanesan J, Ramaswami S, Lakshmanan A, Senthil-Kumar P, Srinivasan UP, Ramasamy R, Ravichandran P. Embolization of liver tumors: past, present and future. World J Radiol. 2012;4:405–412. doi: 10.4329/wjr.v4.i9.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49:523–529. doi: 10.1080/02841850801958890. [DOI] [PubMed] [Google Scholar]
- 10.Xu W, Kwon JH, Moon YH, Kim YB, Yu YS, Lee N, Choi KY, Kim YS, Park YK, Kim BW, Wang HJ. Influence of preoperative transcatheter arterial chemoembolization on gene expression in the HIF-1alpha pathway in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2014;140:1507–1515. doi: 10.1007/s00432-014-1713-4. [DOI] [PubMed] [Google Scholar]
- 11.Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54:1237–1248. doi: 10.1002/hep.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hui L, Chen Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett. 2015;368:7–13. doi: 10.1016/j.canlet.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 13.Critelli R, Milosa F, Faillaci F, Condello R, Turola E, Marzi L, Lei B, Dituri F, Andreani S, Sighinolfi P, Manni P, Maiorana A, Caporali C, di Benedetto F, Del Buono M, De Maria N, Schepis F, Martinez-Chantar ML, Giannelli G, Villa E. Microenvironment inflammatory infiltrate drives growth speed and outcome of hepatocellular carcinoma: a prospective clinical study. Cell Death Dis. 2017;8:e3017. doi: 10.1038/cddis.2017.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HM, Lee E, Yeo SY, Shin S, Park HK, Nam DH, Kim SH. Drug repurposing screening identifies bortezomib and panobinostat as drugs targeting cancer associated fibroblasts (CAFs) by synergistic induction of apoptosis. Invest New Drugs. 2018;36:545–560. doi: 10.1007/s10637-017-0547-8. [DOI] [PubMed] [Google Scholar]
- 15.Petrova V, Annicchiarico-Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7:10. doi: 10.1038/s41389-017-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin Cancer Biol. 2011;21:35–43. doi: 10.1016/j.semcancer.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin TH, Shao YY, Chan SY, Huang CY, Hsu CH, Cheng AL. High serum transforming growth factor-beta1 levels predict outcome in hepatocellular carcinoma patients treated with sorafenib. Clin Cancer Res. 2015;21:3678–3684. doi: 10.1158/1078-0432.CCR-14-1954. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Xiong B, Liang B, Zhao H, Li H, Qian J, Liang HM, Feng GS, Zheng CS. Hepatic parenchymal changes following transcatheter embolization and chemoembolization in a rabbit tumor model. PLoS One. 2013;8:e70757. doi: 10.1371/journal.pone.0070757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Zheng C, Liang B, Zhao H, Qian J, Liang H, Feng G. Hepatocellular necrosis, apoptosis, and proliferation after transcatheter arterial embolization or chemoembolization in a standardized rabbit model. J Vasc Interv Radiol. 2011;22:1606–1612. doi: 10.1016/j.jvir.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Xue H, Lin F, Tan H, Zhu ZQ, Zhang ZY, Zhao L. Overrepresentation of IL-10-expressing B cells suppresses cytotoxic CD4+ T cell activity in HBV-induced hepatocellular carcinoma. PLoS One. 2016;11:e0154815. doi: 10.1371/journal.pone.0154815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fransvea E, Mazzocca A, Antonaci S, Giannelli G. Targeting transforming growth factor (TGF)-betaRI inhibits activation of beta1 integrin and blocks vascular invasion in hepatocellular carcinoma. Hepatology. 2009;49:839–850. doi: 10.1002/hep.22731. [DOI] [PubMed] [Google Scholar]
- 22.Liang B, Chen S, Li L, Liu Y, Xiong F, Dong X, Feng G, Gupta S, Zheng C. Effect of transcatheter intra-arterial therapies on tumor interstitial fluid pressure and its relation to drug penetration in a rabbit liver tumor model. J Vasc Interv Radiol. 2015;26:1879–1886. doi: 10.1016/j.jvir.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 23.Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018;18:128–134. doi: 10.1038/nrc.2017.118. [DOI] [PubMed] [Google Scholar]
- 24.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirota K. Hypoxia-inducible factor 1, a master transcription factor of cellular hypoxic gene expression. J Anesth. 2002;16:150–159. doi: 10.1007/s005400200011. [DOI] [PubMed] [Google Scholar]
- 26.Sheth RA, Hesketh R, Kong DS, Wicky S, Oklu R. Barriers to drug delivery in interventional oncology. J Vasc Interv Radiol. 2013;24:1201–1207. doi: 10.1016/j.jvir.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 27.Pickup M, Novitskiy S, Moses HL. The roles of TGFbeta in the tumour microenvironment. Nat Rev Cancer. 2013;13:788–799. doi: 10.1038/nrc3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawe RT, Kerper M, Badve S, Li J, Sandoval-Cooper M, Xie J, Shi Z, Patel K, Chumba D, Ofulla A, Prosperi J, Taylor K, Stack MS, Mining S, Littlepage LE. Aggressive breast cancer in western Kenya has early onset, high proliferation, and immune cell infiltration. BMC Cancer. 2016;16:204. doi: 10.1186/s12885-016-2204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omyla-Staszewska J, Deptala A. Effective therapeutic management of hepatocellular carcinoma - on the basis of a clinical case. Contemp Oncol (Pozn) 2012;16:60–63. doi: 10.5114/wo.2012.27339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R. EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer. 2009;45:931–991. doi: 10.1016/j.ejca.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 33.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 34.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 35.Moses HL, Roberts AB, Derynck R. The discovery and early days of TGF-beta: a historical perspective. Cold Spring Harb Perspect Biol. 2016;8 doi: 10.1101/cshperspect.a021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tu K, Li J, Verma VK, Liu C, Billadeau DD, Lamprecht G, Xiang X, Guo L, Dhanasekaran R, Roberts LR, Shah VH, Kang N. Vasodilator-stimulated phosphoprotein promotes activation of hepatic stellate cells by regulating Rab11-dependent plasma membrane targeting of transforming growth factor beta receptors. Hepatology. 2015;61:361–374. doi: 10.1002/hep.27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manzoor AA, Lindner LH, Landon CD, Park JY, Simnick AJ, Dreher MR, Das S, Hanna G, Park W, Chilkoti A, Koning GA, ten Hagen TL, Needham D, Dewhirst MW. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012;72:5566–5575. doi: 10.1158/0008-5472.CAN-12-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson CB, Shepard HM, O’Connor PM, Kadhim S, Jiang P, Osgood RJ, Bookbinder LH, Li X, Sugarman BJ, Connor RJ, Nadjsombati S, Frost GI. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther. 2010;9:3052–3064. doi: 10.1158/1535-7163.MCT-10-0470. [DOI] [PubMed] [Google Scholar]
- 39.Whatcott CJ, Han H, Posner RG, Hostetter G, Von Hoff DD. Targeting the tumor microenvironment in cancer: why hyaluronidase deserves a second look. Cancer Discov. 2011;1:291–296. doi: 10.1158/2159-8290.CD-11-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaba RC, Lewandowski RJ, Hickey R, Baerlocher MO, Cohen EI, Dariushnia SR, Janne d’Othée B, Padia SA, Salem R, Wang DS, Nikolic B, Brown DB Society of Interventional Radiology Technology Assessment Committee. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2016;27:457–473. doi: 10.1016/j.jvir.2015.12.752. [DOI] [PubMed] [Google Scholar]
- 41.Habib A, Desai K, Hickey R, Thornburg B, Lewandowski R, Salem R. Transarterial approaches to primary and secondary hepatic malignancies. Nat Rev Clin Oncol. 2015;12:481–489. doi: 10.1038/nrclinonc.2015.78. [DOI] [PubMed] [Google Scholar]
- 42.Lee EW, Khan S. Recent advances in transarterial embolotherapies in the treatment of hepatocellular carcinoma. Clin Mol Hepatol. 2017;23:265–272. doi: 10.3350/cmh.2017.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]