Abstract
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related mortality worldwide and novel therapeutic approaches are urgently required. Anemoside B4 (AB4) is a compound extracted from Pulsatilla chinensis (P. chinensis). Previous studies have indicated that P. chinensis extract P. chinensis saponins has anti-cancer activity. However, the pharmacological effect of AB4 in cancer is largely unknown. In this study, we investigated the anti-cancer efficacy of AB4 in HCC. We used CCK-8 assay and colony formation assay to evaluate the cytotoxicity of AB4 and found that this agent markedly inhibited SMMC7721 cell proliferation. By using a panel of morphological and molecular experiments, we reported that AB4 induced HCC SMMC7721 cell apoptosis and autophagy. Notably, AB4 treatment acts on the Bcl-2-caspase-3 pathway and Beclin-1-LC3-p62 pathway, thereby regulates both apoptosis and autophagy. Finally, we showed that AB4-induced apoptosis and autophagy converges at the PI3K/Akt/mTOR signaling. AB4 treatment inhibits this signaling transduction pathway and leads to HCC cell death. Collectively, our study highlighted the anti-cancer efficacy of AB4 and suggested that AB4 might be a novel way to treat HCC.
Keywords: Anemoside B4, hepatocellular carcinoma, apoptosis, autophagy, PI3K/Akt/mTOR pathway
Introduction
Liver cancer is one of the common malignant tumors with high incidence and mortality according to the latest statistics from Global Cancer 2018 [1]. The types of liver cancer includes hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC) and combined hepatocellular-cholangiocarcinoma (CHC), in which HCC accounts for 70%-90% of cases [2,3]. A number of risk factors for HCC have been identified, such as hepatitis virus infection, smoking, ethanol abuse, and several drugs. Unfortunately, the prognosis of HCC is very poor because most patients are not suitable for surgical resection and chemotherapy remains to be an important approach to treat advanced HCC [4,5]. Although several novel multi-target small molecule inhibitors have been recently approved for treating advanced HCC, the overall response for these drugs are not promising and the patients’ survival are not markedly improved [6]. Thus, there is urgent need for exploring new treatment strategy and therapeutic targets for advanced HCC.
Pulsatilla chinensis (P. chinensis) is a traditional Chinese medicine (TCM) herb that belongs to the buttercup family. It is well known for its “blood-cooling”, detoxification and anti-infectious efficacy and has been used for treating intestinal amebiasis bacterial infections, malaria, vaginal trichomoniasis and cancer in ancient China [7-9]. P. chinensis saponins, a nature triterpenoid glycosides, is the main active component isolated from P. chinensis. Previous studies have reported that P. chinensis saponins has a wide range of pharmacological effects, including anti-inflammatory, anti-oxidant, immunomodulatory and cognitive enhancement [10]. Furthermore, P. chinensis saponins has been used as an anti-tumor drug and for treating hepatitis B in Yichang, Hubei, China [11,12]. However, the pharmacological effect of AB4, a monomeric compound of P. chinensis saponins, is largely unknown.
Autophagy is an evolutionarily-conserved lysosomal pathway, it means that cellular organelles, unfolded proteins and intracellular pathogens were degraded under starvation, oxidative, infection and other stress to meet energy requirements for cell survival [13]. Autophagy includes multiple steps, including initiation, elongation and formation and maturation of double-membrane vesicle termed as autophagosome. Autophagosomes fuse with lysosomes to form autolysosomes for degradation. Autophagy also known as type II programmed cell death and is tightly regulated by autophagy related genes, including ATG, Beclin-1 and p62 [14]. Extensive studies have showed that autophagy plays an important role in HCC tumorigenesis and progression. For example, a number of studies has shown the involvement of autophagy in the pathology of HCC etiologic factors, mainly HBV/HCV infection and alcohol consumption [15]. Autophagy is frequently deregulated in HCC and targeting autophagy would be a novel strategy for advanced HCC. Interestingly, autophagy shows a “double face” in determining cell fate, in which it can either collaborate with apoptosis to promote cell death or sometimes confront with apoptosis [16]. This has led a feasible way to manipulate autophagy to enhance apoptosis or suppress cell death. Both autophagy and apoptosis are regulated by distinct signal transduction pathways, while they shared “common regulatory signalings”, one of these is the PI3K/Akt/mTOR signaling [17]. Previous studies have shown that P. chinensis saponins extracts exert anti-cancer efficacy and induce apoptosis and autophagy by manipulating the PI3K/Akt/mTOR signaling [18]. However, the molecular mechanism that underlies the pharmacological property of AB4 (Figure 1A) remains unclear.
Figure 1.
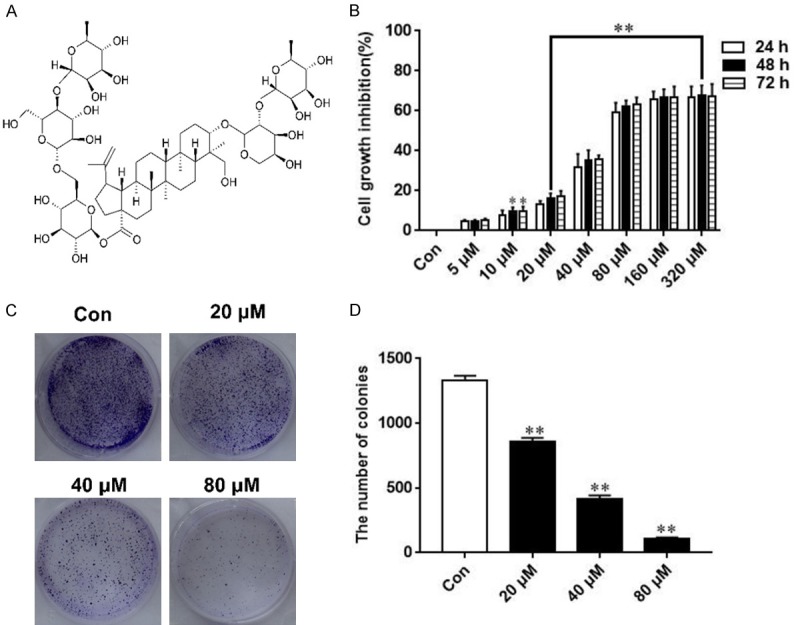
AB4 inhibits SMMC7721 cell proliferation. A. The chemical structures of AB4. B. SMMC7721 cells were treated with ascending concentrations of AB4 (5, 10, 20, 40, 80, 160, 320 μM) for different time periods (24, 48 and 72 h) and cell viability was determined by CCK-8. C and D. Representative images of cell colonies after AB4 treatment. Cells were treated with AB4 (20, 40, 80 μM) for about 14 days, and cell colonies were stained crystal violet and analyzed. **P < 0.01 versus the control group.
In this study, we aimed to investigate potential anti-cancer effect of AB4 in HCC. We showed that both autophagy and apoptosis are involved in the cytotoxicity of AB4 and suggested that inhibition of the PI3K/Akt/mTOR signaling might underlie the molecular machinery of AB4 in cancer treatment.
Material and methods
Reagents
AB4 was purchased from Absin Biotechnology (Shanghai, China). Fetal bovine serum (FBS), Dulbecoo’s Modified Eagle’s Medium (DMEM), Phosphate-Buffered Solution (PBS), trypsin and penicillin-streptomycin solution were purchased from HyClone (Beijing, China). Primary antibodies for Bcl-2, Bax, Cleaved Caspase-3, Cleaved PARP, LC3, Beclin-1, p62, p-Akt, p-mTOR, β-actin were ordered from Cell Signaling Technology (Danvers, MA, USA). The Cell Counting Kit-8 and the Annexin V-FITC Apoptosis Detection Kit were obtained from Beyotime Biotechnology (Beijing, China). Monodansylcadaverine (MDC), Acridine Orange (AO), chloroquine (CQ), and dimethyl sulfoxide (DMSO) were obtained from Sigma (St. Louis, MO, USA).
Cell culture
The SMMC7721 cells were purchased from the Shanghai Institutes of Biological Sciences. Cells were cultured in DMEM supplement with 10% FBS and penicillin-streptomycin (100 U/mL). Cells were incubated in a humidified incubator with 5% CO2 at 37°C.
CCK-8 assay
Cells were seeded into 96 well-plate at a density of 5 × 103 per well, then treated with ascending concentrations of AB4 (5, 10, 20, 40, 80, 160, 320 μM) for different time periods (24, 48 and 72 h). Cells were subsequently incubated with 10 μL CCK-8 for additional 1 h. The optical density (OD) at 470 nm was determined by microplate reader (Bio-Tek, VT, USA).
Colony formation assay
Cells were seeded into 6 well-plate at a density of 1 × 103 per well, and treated with AB4 (20, 40, 80 μM) for about 14 days. The medium was discarded and the cells were washed with PBS three times. After being fixed with 4% paraformaldehyde for 20 min at room temperature, the cell colonies were stained with 1% crystal violet for 15 min. Finally, the dye was washed with PBS, the colonies in each well were quantified and photographed.
MDC and AO staining
Cells were seeded in 6-well plates and incubated with 80 μM AB4. After 24 h of treatment, cells were stained with 50 μM MDC or AO solution for 30 min. The MDC and AO fluorescence was observed under a confocal laser scanning microscope (Olympus, Tokyo, Japan) and fluorescent microscope (Olympus, Tokyo, Japan), respectively.
Western blot
Protein were extracted from AB4-treated and vehicle-treated cells. Protein samples were extracted with RIPA buffer containing protease inhibitor cocktail. Protein quantified was performed using a BCA protein assay kit. Protein samples were separated using 10% SDS-PAGE and then transferred to PVDF membranes. Then membranes were blocked with 5% BSA in TBST for 1 h and incubated with corresponding primary antibodies overnight at 4°C. The membranes were incubated with an secondary antibody for 1 h at room temperature. The protein bands were detected by enhanced chemiluminescence kit (Millipore, MA, USA).
Immunofluorescent staning and Flow cytometry assay
Cells were seeded into 6 well-plate at a density of 2 × 105 per well, treated with 80 μM AB4 or DMSO for 24 h and diluted to indicated concentration. Cells were suspended in 195 μL of Annexin V-FITC binding buffer, incubated with with 5 μL Annexin-V-FITC and 10 μL PI for 25 min at 4°C. The cell fluorescence and apoptosis were detected by confocal laser scanning microscope and flow cytometer, respectively.
Transmission electron microscopy
After indicated treatment, cells were fixed overnight at 4°C in 2% paraformaldehyde, 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer overnight at 4°C, before being post-fixed with 1% OSO4 for 1 h. Cells were then dehydrated in a graded ethanol series and embedded in agar 100 epoxy resin. Ultrathin sections were mounted on Cu grids and stained first with uranyl acetate followed by lead citrate. The sections were observed and photographed under transmission election microscope (Joel, JEM-2000EX, Tokyo, Japan).
Statistical analysis
All experiments were repeated independently at least 3 times. Data were presented as mean ± SD. Statistical analysis were carried out using SPSS 20.0 software (Chicago, IL, USA). Paired t test and one-way analysis of variance (ANOVA) were employed to determine the statistical significance between different groups. Significant difference was set at *P < 0.05 and **P < 0.01.
Results
AB4 inhibits the proliferation of SMMC7721 cells
We investigate the effect of AB4 on SMMC7721 cell growth by evaluating cell viability using CCK-8 analysis. SMMC7721 cells were treated with 5, 10, 20, 40, 80, 160, 320 μM for 24, 48 and 72 h, respectively. AB4 inhibition was observed in 24 h phase, and it continued to increase over the next 72 h. As shown in Figure 1B, AB4 inhibited SMMC7721 cell proliferation in dose- and time-dependent manner.
The cytotoxicity of AB4 on SMMC7721 cells at a relatively long period has been confirmed by colony formation assay (Figure 1C and 1D). AB4 at all the tested concentration markedly prohibited SMMC7721 colony number on the cell culture plate, and this inhibitory effect was significant compared to the vehicle control group. These results suggested that AB4 is a candidate for treating advanced HCC.
AB4 induces apoptosis in SMMC7721 cells
To underlie the molecular mechanism of the cytotoxicity of AB4, we therefore investigated whether this agent induces apoptosis. We used immunofluorescent staining to evaluate apoptosis because the expression and distribution of Annexin V and PI various in normal and apoptotic cells. As shown in Figure 2A, treatment with AB4 readily triggered SMMC7721 cell apoptosis, as judged by Annexin V and PI staining. In contrast, cells treated with vehicle control did not exhibited apoptotic immunofluorescent patterns.
Figure 2.
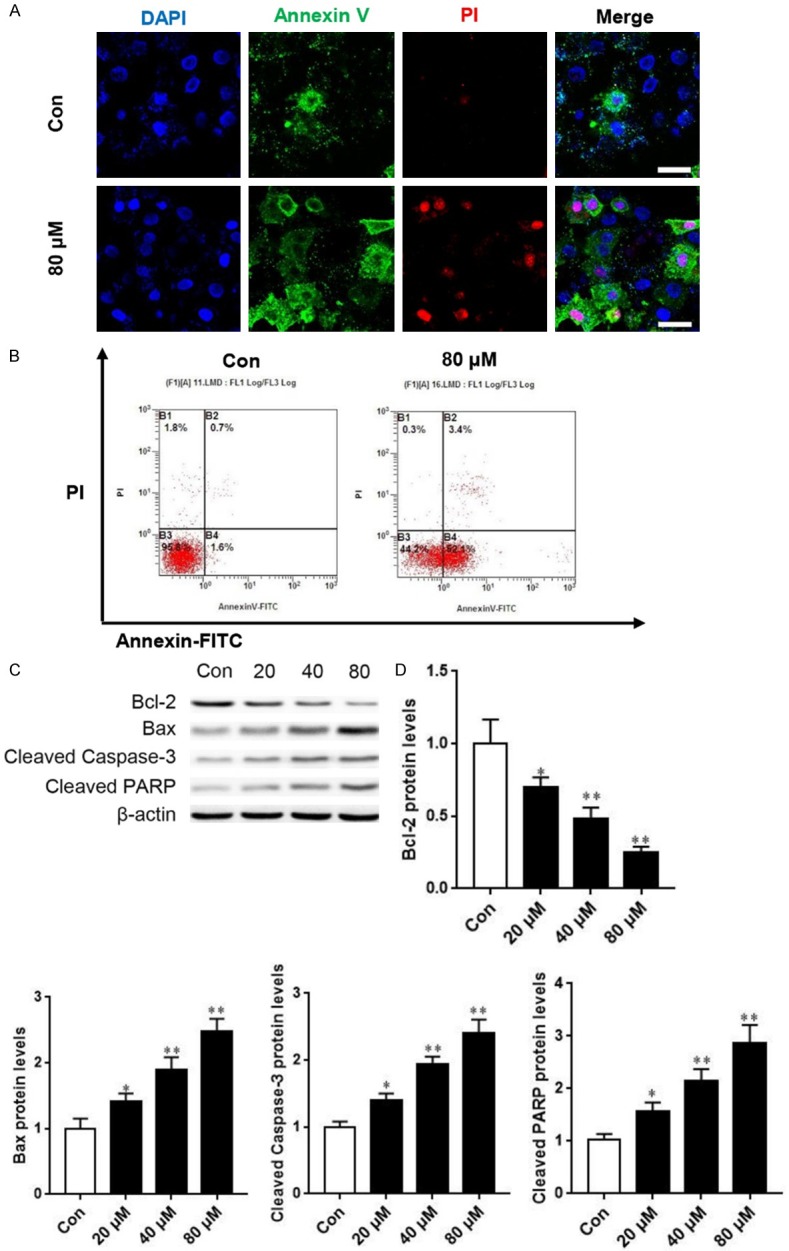
AB4 induces SMMC7721 cell apoptosis. A. Representative images of DAPI, Annexin V-FITC and PI fluorescence staining. Scale bar = 10 μm. B. Flow cytometry analysis of apoptosis after treatment with 80 μM AB4 or vehicle control. C. SMMC7721 cells were treated with 20, 40 and 80 μM AB4 for 24 h. Western blot analysis was performed to evaluate the expression of apoptosis-related proteins. D. Grayscale analysis of Bcl-2, Bax, Cleaved Caspase-3 and Cleaved PARP after indicated treatment. *P < 0.05, **P < 0.01 versus vehicle control group.
We also used flow cytometry to determine cell apoptosis. Cells showed four different cell populations marked as: dead cells (B1), late apoptosis (B2), live cell population (B3) and early apoptosis (B4). Consistent with our previous findings, AB4 treatment readily induced SMMC7721 cell apoptosis (Figure 2B). In further support of this notion, Western blot analysis of apoptosis-related proteins showed that AB4 treatment inhibited the expression of anti-apoptotic protein Bcl-2, whereas increased pro-apoptotic Bax protein level, Moreover, AB4 treatment induced cleaved caspase-3 and PARP expression (Figure 2C and 2D). These findings strongly indicated that apoptosis is involved in the anti-cancer efficacy of AB4 in HCC, treatment with this agent promotes pro-apoptotic protein expression and thereby triggers apoptosis.
AB4 triggers autophagy in SMMC7721 cells
Similar to apoptosis, autophagy is also an important form of programmed cell death. Apoptosis and autophagy could occur simultaneously and sometimes cooperate with each other to promote cell death. To determine whether AB4 induces autophagy, we first detected morphological changes of SMMC7721 cells after AB4 treatment under TEM. As we have mentioned earlier, the formation of double membrane autophagic vacuoles is a hallmark of autophagy, these autophagic vacuoles could be readily detected in cells treated with AB4, but not in cells treated with vehicle control (Figure 3A). Autophagic cells also exhibited other morphological features, including accumulation of MDC-labeled vesicles (Figure 3B and 3C) and acidic autophagosomes in AB4-treated cells compared to vehicle-treated control cells (Figure 3D and 3E).
Figure 3.
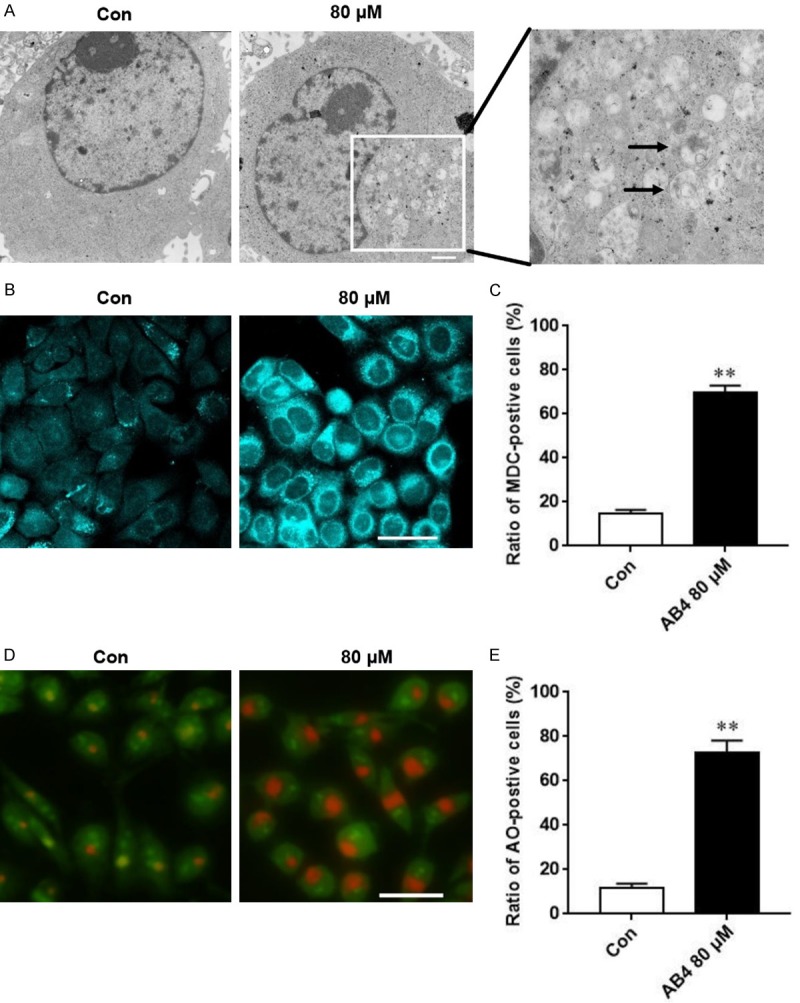
AB4 treatment induces cell autophagy. A. Representative images of autophagosome in SMMC7721 cells after 80 μM AB4 treatment. Scale bar = 500 nm. B and C. Representative images and quantitative analysis of MDC fluorescent staining showing SMMC7721 cells undergoing auophagy. Scale bar = 30 μm. D and E. Representative images and quantitative analysis of AO fluorescent staining showing SMMC7721 cells undergoing auophagy. Scale bar = 30 μm.
In the analysis of autophagy-related proteins, AB4 treatment dose-dependently resulted in autophagy flux, such as LC3-II/LC3-I ratio and p62 protein degradation. Furthermore, expression of autophagy biomarker Beclin-1 also reflected the induction of autophagy after AB4 treatment (Figure 4A and 4B). These autophagy-inducing effect of AB4 could be blocked by an autophagy inhibitor, CQ, as co-treatment with CQ readily prohibited autophagy flux and p62 degradation (Figure 4C and 4D). These data strongly indicated that AB4 induced autophagy in SMMC7721 cells.
Figure 4.
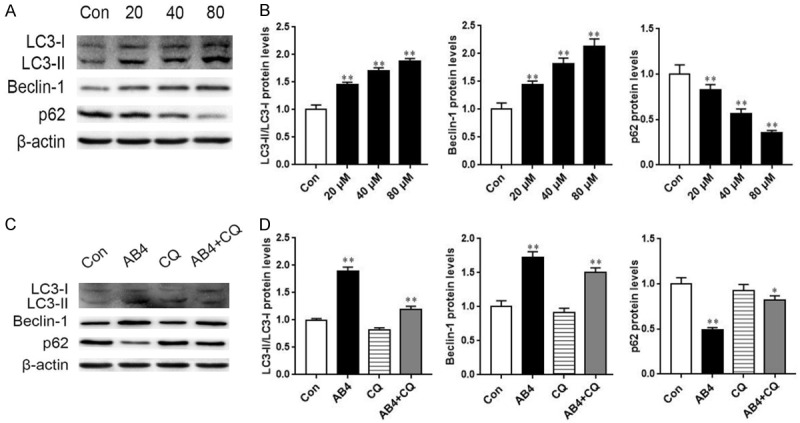
Western blot analysis of autophagy-related proteins after AB4 treatment. A and B. SMMC7721 cells were treated with 20, 40 and 80 μM AB4 for 24 h. Western blot analysis was performed to analysis the expression of autophagy-related proteins, including LC3, Beclin-1 and p62. β-actin was used for equal loading. *P < 0.05, **P < 0.01 versus vehicle control group. C and D. SMMC7721 cells were pre-treated with 20 μM CQ for 1 h, followed by 24 h of AB4 treatment. Autophagy-related proteins were detected by Western blot and quantitatively analyzed. *P < 0.05, **P < 0.01 versus vehicle control group.
AB4 inhibited PI3K/Akt/mTOR signaling
To explore the mechanism by which AB4 induces apoptosis and autophagy, we examined signaling upstream of these two programmed cell death pathways. As shown in Figure 5, AB4 treatment inhibited the phosphorylation level of Akt, while had no effect on total Akt protein level. Similarly, the phosphorylation of mTOR could be reduced by AB4 treatment, suggesting that AB4 inhibited the PI3K/Akt/mTOR pathway.
Figure 5.
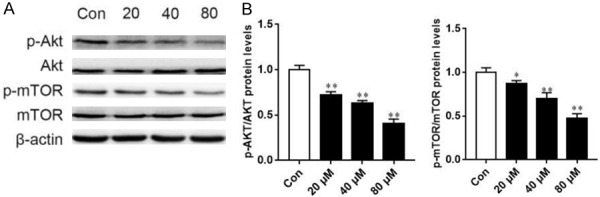
AB4 inhibits the PI3K/Akt/mTOR pathway signaling. A. SMMC7721 cells were treated with 20, 40 and 80 μM AB4 for 24 h. Phosphorylation of Akt and mTOR was determined by Western blot analysis. B. Grayscale analysis of Akt and mTOR phosphorylation after AB4 treatment. *P < 0.05, **P < 0.01 versus vehicle control group.
Discussion
Currently, increasing natural occurring drugs are used for adjuvant treatment of HCC because of their safety and minimal adverse effect. These natural occurring drugs or their chemical modified derivates have been shown to inhibit HCC cell proliferation and induce cancer cell apoptosis [19]. More importantly, they have sensitizing efficacy when used in combination with chemotherapeutic agents [20]. In the present study, we reported the anti-cancer effect of P. chinensis extract, AB4, in HCC. We showed that AB4 leads to HCC programmed cell death and inhibits the PI3K/Akt/mTOR signaling, suggesting that AB4 might be a novel therapeutic strategy for advanced HCC.
Apoptosis is caused by pro-apoptotic signals such as DNA damage and loss of growth factors. Caspase-3 is a major executor of apoptosis, which can specifically cleave substrates such as PARP, that eventually leads to DNA fragmentation and cell apoptosis. The Bcl-2 family is tightly involved in caspase-3-mediated apoptosis [21,22]. Bax acts as a pro-apoptotic protein that promotes the release of Cytochrome C into mitochondria and activates caspase-3 [23]. In contrast, Bcl-2 protein antagonizes Bax and inhibits the initiation of apoptosis [24,25]. In our study, we found that AB4 readily inhibited HCC SMMC7721 cell proliferation, colony formation and induced apoptosis, as judged by flow cytometry and Western blot. These results indicated that AB4 has anti-cancer activity in HCC, in which AB4 induces apoptosis through manipulating the Bcl-2 family.
Interestingly, autophagy as another important form of programmed cell death is frequently deregulated in cancer. While autophagy has both cell death promoting and cell death inhibiting activity, which largely depends on cell types and the magnitude of autophagy [19,26]. Platinum and other chemotherapeutic drugs have been shown to induce HCC autophagy and the significance of autophagy in this setting is still controversial [27]. We showed that AB4 is also capable of inducing autophagy at all the tested concentration. We confirmed autophagy by TEM and other morphological experiment. When autophagy occurs, Beclin-1 binds to the VPS34 complex that participates in the vesicle nucleation process. The proLC3 is processed into LC3-1 and LC3-1 reversibly binds to the hydroxyl end of phosphatidylethanolamine, forming LC3-II on the surface of autophagosome. During this process, p62 is a key autophagic substrate that integrates LC3 into autophagosomes. The fusion of autophagosomes and lysosomes leads to substrate degradation by lysosomal enzymes. In our study, we also found the Belin-1-LC3-p62 cascade, in which AB4 treatment resulted in Bclin-1 accumulation, LC3 flux and p62 degradation. Moreover, we found that the autophagy inhibitor CQ could reverse AB4-induced cell autophagy. These molecular changes might highlight the mechanism of AB4-induced autophagy in HCC. Given that AB4 induces both apoptosis and autophagy, an interesting question has raised: What is the significance of the two types of programmed cell death? As autophagy is a two-side sword, it can either promote cell survival against nutrition deprivation or lead to autophagic cell death. Our ongoing study suggested that inhibition of autophagy by CQ impaired SMMC7721 cell apoptosis regardless of AB4 concentration (unpublished data). Therefore, we hypothesized that AB4-induced autophagy is a pro-apoptotic molecular event.
Finally, we investigated the signaling pathway upstream of apoptosis and autophagy that would be responsible for AB4’s anti-cancer efficacy. We demonstrated that apoptosis and autophagy converges at the PI3K/Akt/mTOR signaling. Phosphorylation of PI3K and Akt is two important mediators of apoptosis, and mTOR is the major negative regulator of autophagy and is located downstream of the pathway. Thus, targeting PI3K/Akt/mTOR not only triggers apoptosis, but also induces autophagy [28]. And inhibiting is PI3K/Akt/mTOR signaling a promising way to treat cancer [29]. Indeed, numerous anti-cancer drugs have been proved to inhibit this pathway and mutations in PIK3CA and mTOR are associated with carcinogenesis and chemotherapy resistance [30]. We found that AB4 inhibited Akt and mTOR phosphorylation, thereby inducing both apoptosis and autophagy. We also noted that AB4 could not totally block Akt and mTOR phosphorylation, thus, we anticipated that AB4 in combination with chemotherapy agents may totally abolish the PI3K/Akt/mTOR signaling and kill cancer cells more efficiently.
Taken together, this study demonstrates that AB4 can inhibit the proliferation of HCC SMMC-7721 and induce apoptosis and autophagy. Inactivation of the PI3K/Akt/mTOR signaling might be the molecular basis for AB4’s anti-cancer efficacy. Further studies evaluating potential chemosensitizing effect and its safety in HCC patients are urgently needed.
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Han G, Zhang L, Ni X, Chen Z, Pan X, Zhu Q, Li S, Wu J, Huang X, Wang X. MicroRNA-873 promotes cell proliferation, migration, and invasion by directly targeting TSLC1 in hepatocellular carcinoma. Cell Physiol Biochem. 2018;46:2261–2270. doi: 10.1159/000489594. [DOI] [PubMed] [Google Scholar]
- 3.Teng YC, Shen ZQ, Kao CH, Tsai TF. Hepatocellular carcinoma mouse models: Hepatitis B virus-associated hepatocarcinogenesis and haploinsufficient tumor suppressor genes. World J Gastroenterol. 2016;22:300–325. doi: 10.3748/wjg.v22.i1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao X, Chen Q, Liu W, Li Y, Tang H, Liu X, Yang X. Codelivery of doxorubicin and curcumin with lipid nanoparticles results in improved efficacy of chemotherapy in liver cancer. Int J Nanomedicine. 2015;10:257–270. doi: 10.2147/IJN.S73322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Y, Lv SX. The effect of JAK2 knockout on inhibition of liver tumor growth by inducing apoptosis, autophagy and anti-proliferation via STATs and PI3K/AKT signaling pathways. Biomed Pharmacother. 2016;84:1202–1212. doi: 10.1016/j.biopha.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 6.Elsegood CL, Tirnitz-Parker JE, Olynyk JK, Yeoh GC. Immune checkpoint inhibition: prospects for prevention and therapy of hepatocellular carcinoma. Clin Transl Immunology. 2017;6:e161. doi: 10.1038/cti.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong X, Han L, Duan H, Cui Y, Feng Y, Zhu Y, Chen Z, Yang S. The derivatives of Pulsatilla saponin A, a bioactive compound from Pulsatilla chinensis: Their synthesis, cytotoxicity, haemolytic toxicity and mechanism of action. Eur J Med Chem. 2017;129:325–336. doi: 10.1016/j.ejmech.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Cheng L, Zhang M, Zhang P, Song Z, Ma Z, Qu H. Silver complexation and tandem mass spectrometry for differentiation of triterpenoid saponins from the roots of Pulsatilla chinensis (Bunge) Regel. Rapid Commun Mass Spectrom. 2008;22:3783–3790. doi: 10.1002/rcm.3801. [DOI] [PubMed] [Google Scholar]
- 9.Xu QM, Shu Z, He WJ, Chen LY, Yang SL, Yang G, Liu YL, Li XR. Antitumor activity of Pulsatilla chinensis (Bunge) Regel saponins in human liver tumor 7402 cells in vitro and in vivo. Phytomedicine. 2012;19:293–300. doi: 10.1016/j.phymed.2011.08.066. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Ji X, Shi X, Du Y, Zhu H, Zhang L. Development of a novel method for triterpenoidal saponins in rat plasma by solid-phase extraction and high-performance liquid chromatography tandem mass spectrometry. Anal Biochem. 2011;419:323–332. doi: 10.1016/j.ab.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 11.Guo X, Xie Y, Lian S, Li Z, Gao Y, Xu Z, Hu P, Chen M, Sun Z, Tian X, Huang C. A sensitive HPLC-MS/MS method for the simultaneous determination of anemoside B4, anemoside A3 and 23-hydroxybetulinic acid: Application to the pharmacokinetics and liver distribution of Pulsatilla chinensis saponins. Biomedical Chromatography. 2018;32:e4124. doi: 10.1002/bmc.4124. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y, Zhou F, Wu X, Wen X, Li Y, Yan B, Zhang J, Hao G, Ye W, Wang G. 23-Hydroxybetulinic acid from Pulsatilla chinensis (Bunge) Regel synergizes the antitumor activities of doxorubicin in vitro and in vivo. J Ethnopharmacol. 2010;128:615–622. doi: 10.1016/j.jep.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Fu XT, Shi YH, Zhou J, Peng YF, Liu WR, Shi GM, Gao Q, Wang XY, Song K, Fan J, Ding ZB. MicroRNA-30a suppresses autophagy-mediated anoikis resistance and metastasis in hepatocellular carcinoma. Cancer Lett. 2018;412:108–117. doi: 10.1016/j.canlet.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Cui Q, Tashiro S, Onodera S, Minami M, Ikejima T. Autophagy preceded apoptosis in oridonin-treated human breast cancer MCF-7 cells. Biol Pharm Bull. 2007;30:859–864. doi: 10.1248/bpb.30.859. [DOI] [PubMed] [Google Scholar]
- 15.Ding WX. Role of autophagy in liver physiology and pathophysiology. World J Biol Chem. 2010;1:3–12. doi: 10.4331/wjbc.v1.i1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Metivier D, Meley D, Souquere S, Yoshimori T, Pierron G, Codogno P, Kroemer G. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Zhu M, Wang Q, Hou Y, Li L, Weng H, Zhao Y, Chen D, Ding H, Guo J, Li M. Alpha-fetoprotein inhibits autophagy to promote malignant behaviour in hepatocellular carcinoma cells by activating PI3K/AKT/mTOR signalling. Cell Death Dis. 2018;9:1027. doi: 10.1038/s41419-018-1036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Son MK, Jung KH, Hong SW, Lee HS, Zheng HM, Choi MJ, Seo JH, Suh JK, Hong SS. SB365, Pulsatilla saponin D suppresses the proliferation of human colon cancer cells and induces apoptosis by modulating the AKT/mTOR signalling pathway. Food Chem. 2013;136:26–33. doi: 10.1016/j.foodchem.2012.07.096. [DOI] [PubMed] [Google Scholar]
- 19.Ye R, Dai N, He Q, Guo P, Xiang Y, Zhang Q, Hong Z, Zhang Q. Comprehensive anti-tumor effect of Brusatol through inhibition of cell viability and promotion of apoptosis caused by autophagy via the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomed Pharmacother. 2018;105:962–973. doi: 10.1016/j.biopha.2018.06.065. [DOI] [PubMed] [Google Scholar]
- 20.Chang YT, Wang CCN, Wang JY, Lee TE, Cheng YY, Morris-Natschke SL, Lee KH, Hung CC. Tenulin and isotenulin inhibit P-glycoprotein function and overcome multidrug resistance in cancer cells. Phytomedicine. 2019;53:252–262. doi: 10.1016/j.phymed.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou P, Chou J, Olea RS, Yuan J, Wagner G. Solution structure of Apaf-1 CARD and its interaction with caspase-9 CARD: a structural basis for specific adaptor/caspase interaction. Proc Natl Acad Sci U S A. 1999;96:11265–11270. doi: 10.1073/pnas.96.20.11265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim C, Kim B. Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: a review. Nutrients. 2018;10 doi: 10.3390/nu10081021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Wang H, Ge H, Yang Z. AG-1031 induced autophagic cell death and apoptosis in C6 glioma cells associated with Notch-1 signaling pathway. J Cell Biochem. 2018;119:5893–5903. doi: 10.1002/jcb.26781. [DOI] [PubMed] [Google Scholar]
- 24.Kiraz Y, Adan A, Kartal Yandim M, Baran Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumour Biol. 2016;37:8471–8486. doi: 10.1007/s13277-016-5035-9. [DOI] [PubMed] [Google Scholar]
- 25.Mukhopadhyay S, Panda PK, Sinha N, Das DN, Bhutia SK. Autophagy and apoptosis: where do they meet? Apoptosis. 2014;19:555–566. doi: 10.1007/s10495-014-0967-2. [DOI] [PubMed] [Google Scholar]
- 26.Sun X, Li L, Ma HG, Sun P, Wang QL, Zhang TT, Shen YM, Zhu WM, Li X. Bisindolylmaleimide alkaloid BMA-155Cl induces autophagy and apoptosis in human hepatocarcinoma HepG-2 cells through the NF-kappaB p65 pathway. Acta Pharmacol Sin. 2017;38:524–538. doi: 10.1038/aps.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J, Guo J, Cao Q, Wang Y, Chen J, Wang Z, Yuan Z. Autophagy impacts on oxaliplatin-induced hepatocarcinoma apoptosis via the IL-17/IL-17R-JAK2/STAT3 signaling pathway. Oncol Lett. 2017;13:770–776. doi: 10.3892/ol.2016.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu F, Gao S, Yang Y, Zhao X, Fan Y, Ma W, Yang D, Yang A, Yu Y. Antitumor activity of curcumin by modulation of apoptosis and autophagy in human lung cancer A549 cells through inhibiting PI3K/Akt/mTOR pathway. Oncol Rep. 2018;39:1523–1531. doi: 10.3892/or.2018.6188. [DOI] [PubMed] [Google Scholar]
- 29.Zhou L, Huang Y, Li J, Wang Z. The mTOR pathway is associated with the poor prognosis of human hepatocellular carcinoma. Med Oncol. 2010;27:255–261. doi: 10.1007/s12032-009-9201-4. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Nie H, Zhao X, Qin Y, Gong X. Bicyclol induces cell cycle arrest and autophagy in HepG2 human hepatocellular carcinoma cells through the PI3K/AKT and Ras/Raf/MEK/ERK pathways. BMC Cancer. 2016;16:742. doi: 10.1186/s12885-016-2767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


