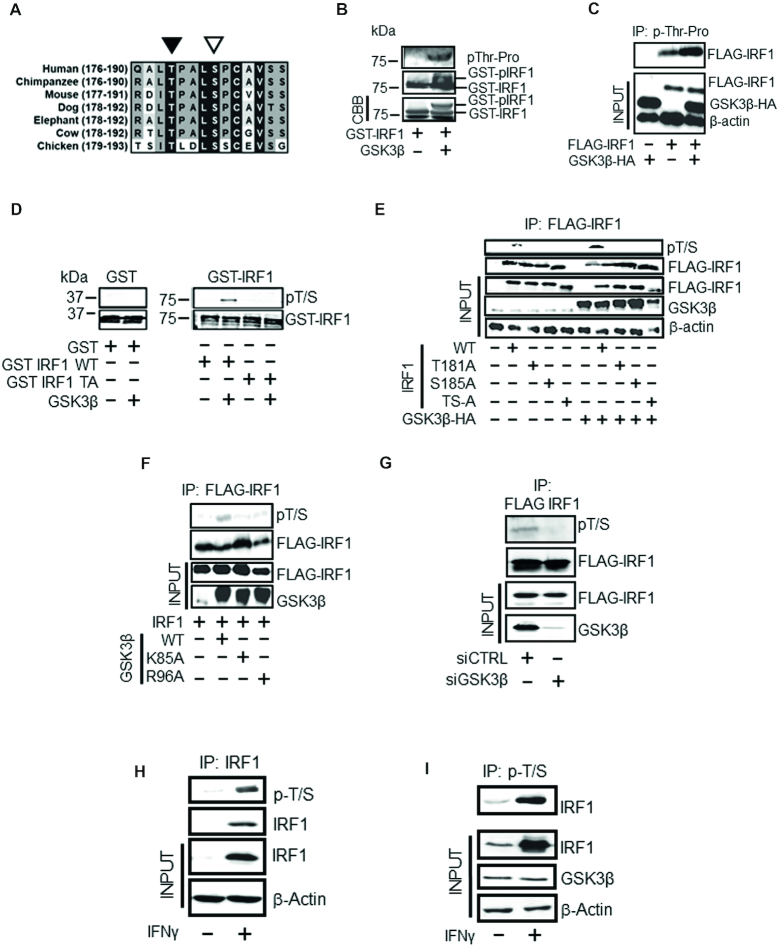
Figure 1.
IRF1 is phosphorylated by GSK3β. (A) Sequence conservation of the putative GSK3β phospho-target sequence in different species. The phosphorylated T180 and the +4 priming site (S184) (T181/S185 in murine sequence) residues are indicated by black and white arrowheads, respectively. Residue numbers in parentheses. (B) In vitro kinase assay performed using recombinant GSK3β and purified GST-IRF1 protein as substrate. The reaction products were resolved by SDS PAGE and GST-IRF1 T181 phosphorylation revealed by western blotting using anti-pTP antibody (top panel). Altered migration of GST-IRF1 after phosphorylation by GSK3β was also visible after western blot detection using anti-IRF1 (middle panel), or by Coomassie brilliant blue (CBB) staining (bottom panel). The lowest band in the CBB panel is the loading dye front. (C) Lysates from HEK293 cells expressing GSK3β-HA and mouse FLAG-IRF1, immunoprecipitated with the p-TP antibody. Immunoprecipitated IRF1 was detected with anti-FLAG antibody. Inputs (10%) indicate the expression of transfected FLAG-IRF1 and GSK3β-HA proteins, and loading control β-actin. (D) In vitro kinase assay performed as in 1B but visualized by immunoblot with pT/S (pThr58/Ser62 c-Myc) and with GST tag control. The T181A mutant is included to demonstrate specificity of the antibody. Note: the GST and GST-IRF1 samples were run in parallel on separate SDS-PAGE gels. (E) Lysates of HEK293 cells expressing FLAG-IRF1 WT or mutants together with GSK3β-HA or empty vector were immunoprecipitated with anti-FLAG beads. IRF1 T181/S185 dual phosphorylation was detected by western blotting with pT/S antibody (top panel). Successful IP of IRF1 proteins in the extracts was confirmed by re-probing with anti-FLAG antibody (second panel). Inputs (10%) are shown in the lower three panels and indicate the levels of IRF1 (anti-FLAG), GSK3β (anti-HA) and loading control β-actin. (F) As for E), but with GSK3β kinase inactive (K85A) and priming mutants (R96A). (G) HEK293 cells treated with siRNAs to deplete GSK3β (or control) for 24 hr prior to transfection with FLAG-IRF1 for a further 48 hr. Lysates were immunoprecipitated and probed with pT/S antibody and FLAG to show IP efficiency. (H) Extracts from MRC5 cells treated for 3 hr with IFNγ (1000U / mL) or vehicle were immunoprecipitated with anti-IRF1 and probed with pT/S. Input lysates (10%) are shown below. (I) H3396 lysates (IFNγ treated as for H) immunoprecipitated with pT/S followed by probe with IRF1. Input lysates (10%) are shown below against IRF1, GSK3β and β-actin.