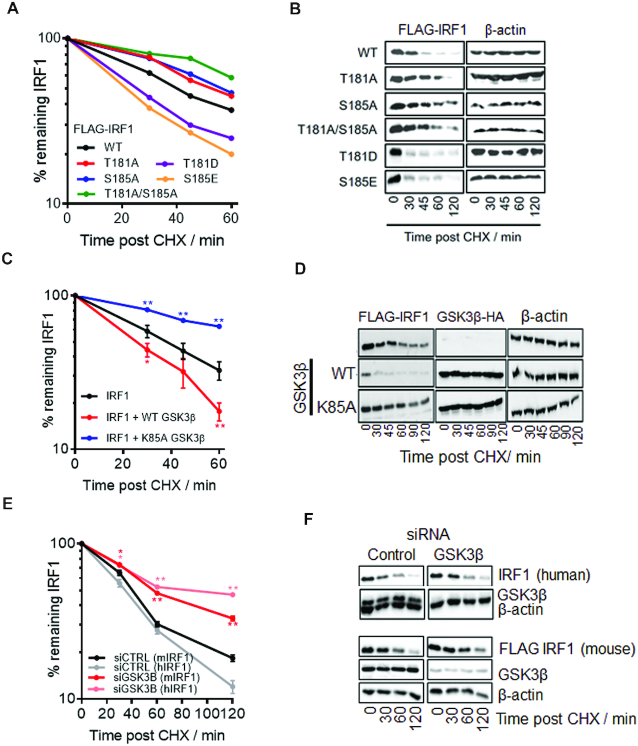
Figure 6.
Phosphorylation of T181/S185 by GSK3β promotes IRF1 degradation. (A) Cycloheximide (CHX) chase to detect turnover of IRF1 proteins. MRC5 cells expressing IRF1 WT or mutants were treated with CHX to prevent further protein synthesis. Whole cell extracts were prepared at the times indicated post CHX treatment and subjected to western blotting, using anti-IRF1 antibody. IRF1 expression was quantified using densitometry (ImageJ) and expressed relative to β-actin levels; untreated was set at 100%. Data is from three independent experiments performed in duplicate. Also see Supplementary Figure S3A for t-test significance. (B) Western blot of IRF1 CHX chases related to (A). (C) CHX chase in MRC5 cells expressing IRF1 and GSK3β, calculated as for (A). Error bars = s.e.m., Student's t-test shows significance between empty vector + IRF1 and GSK3β + IRF1. (D) Western blot of IRF1 and GSK3β related to (D). (E) MRC5 cells transfected with controL (siCTRL) or GSK3β siRNAs (10 nM) for 24 h followed by transfection with FLAG-IRF1 for 48 hr. Parallel siRNA transfected samples were treated with IFNγ (1000 U/ml 3 h) to induce endogenous IRF1 expression and subjected to CHX chase for indicated times. Lysates were probed with FLAG to detect exogenous IRF1 and IRF1 C20 (human specific) antibody to detect the endogenous IRF1. Error bars = s.e.m., Student's t-test shows significance between siCTRL and siGSK3β for mouse and human IRF1. (F) Western blots from (E) probed for FLAG (exogenous mouse IRF1) and human IRF1 (C20 antibody is non-cross reactive with murine IRF1), GSK3β (knockdown efficiency) and β-actin loading control.