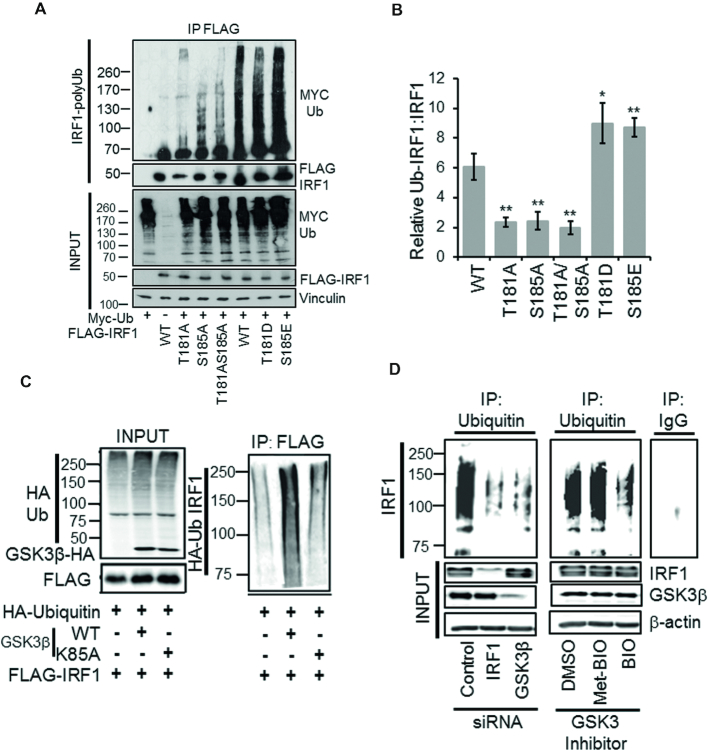
Figure 7.
GSK3β promotes IRF1 ubiquitination. (A) Ubiquitination of IRF1; HEK293 cells expressing FLAG-IRF1, and MYC-Ub were treated with MG132 (10 μM) for 6 h prior to IP for FLAG-IRF1 and probe with myc (Ub-IRF1). Input lysates were probed with anti-FLAG, anti-myc and anti-vinculin. (B) Quantification of relative levels of ubiquitination of IRF1 proteins. Data is expressed as the relative levels of the IRF1-Ub species versus the IRF1 from inputs (to account for differences in expression). Data is from three experiments. Error bars denote SEM. Significant differences were determined by Student's t-test comparing WT to each mutant. (C) HEK293 cells expressing HA-Ub, FLAG-IRF1 WT, GSK3β-HA WT and GSK3β -HA K85A were lysed 48 hr post transfection in SDS denaturing buffer, boiled and diluted 10-fold in PBS and immunoprecipitated with FLAG. The resulting high molecular weight Ub modified IRF1 was detected by HA western blot. Input panel shows expression of transfected proteins. (D) Ubiquitin immunoprecipitation of endogenous IRF1 in MRC5 lysates from cells siRNA depleted of IRF1 or GSK3β, or pre-treated with GSK3 Inhibitor BIO, or its inactive analog met-BIO (10 μM for 1 h). MG132 (10 μM for 5 h) was added prior to lysis to prevent degradation of ubiquitinated IRF1. Ub-IRF1 smears were detected by blot against human IRF1 using the C-20 antibody. Knockdown efficiencies for IRF1 and GSK3β are shown in the input panel. Control IgG immunoprecipitation is shown on the adjacent panel.