Figure 9.
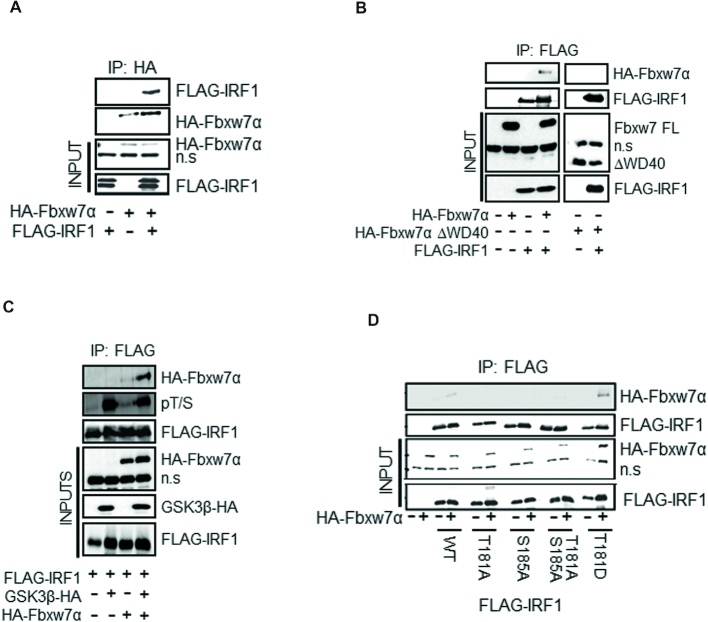
Fbxw7α interacts with phosphorylated IRF1 via T181. (A) Co-immunoprecipitation of HA-Fbxw7α in extracts of HEK293 cells, and western blots using anti-HA antibody to detect associated FLAG-IRF1. Cells were treated with MG132 for 6 h prior to lysis. Blots were re-probed with anti-HA to determine IP efficiency n.s. = non-specific band is indicated. (B) Co-IP experiment as in (A) but with anti-FLAG antibody to IP and western blot with anti-HA to detect complex formation with HA-Fbxw7α or a mutant lacking the WD40 domain (HA-Fbxw7α ΔWD40). (C) HA-Fbxw7α, GSK3β-HA and FLAG-IRF1 were co-expressed in HEK293 for 48 h, 6 h prior to lysis cells were treated with 10 μM MG132. Lysates were immunoprecipitated with anti-FLAG and probed with anti-HA to detect Fbxw7α interaction. The blots were also probed with the anti-pT/S antibody and FLAG to determine relative levels of IRF1 phosphorylation. (D) Immunoprecipitations as for (B) but with the inclusion of IRF1 T181A, S185A, T181A/S185A and T181D mutants.