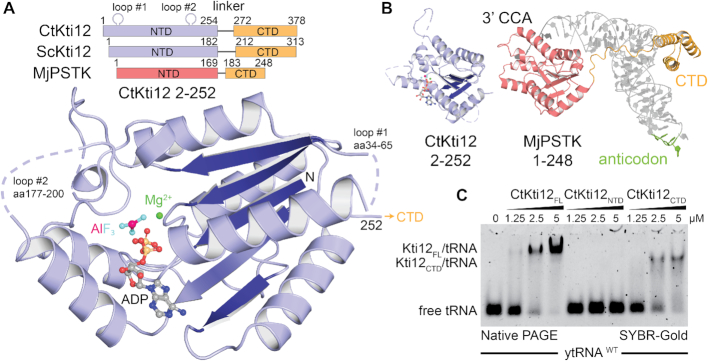
Figure 1.
Kti12 resembles PSTK both structurally and functionally. (A) Structure of a CtKti12NTD locked in a transition state of ATP hydrolysis shown in a cartoon representation. Aluminum fluoride, a hydrolysis transition state mimetic (magenta and cyan) and magnesium (green sphere) are highlighted. The protein core consists of a single β-sheet composed of five parallel β-strands (dark blue). Domain architectures of CtKti12 and ScKti12 are similar to that of MjPSTK. ATPase motifs located on the N-terminal domain (NTD) of Kti12 (blue) and PSTK (red); C-terminal domain (CTD; yellow). (B) Structure of CtKti12NTD (blue) resembles the one of MjPSTKNTD bound to tRNASec (red; PDB ID 3ADB). The 3′ CCA of tRNASec is inserted into the PSTKNTD while the tRNA body is mainly held by PSTKCTD (yellow). The anticodon region (green) is not involved in this interaction. C. Binding affinity of CtKti12 with bulk yeast tRNA was examined by electrophoretic mobility shift assay (EMSA) using 5% native PAGE. Free tRNA and tRNA in complex with CtKti12-FL or CtKti12CTD were visualized using SYBR-Gold. Both full-length (FL) protein and CtKti12CTD bind tRNA in a PSTK-like fashion.