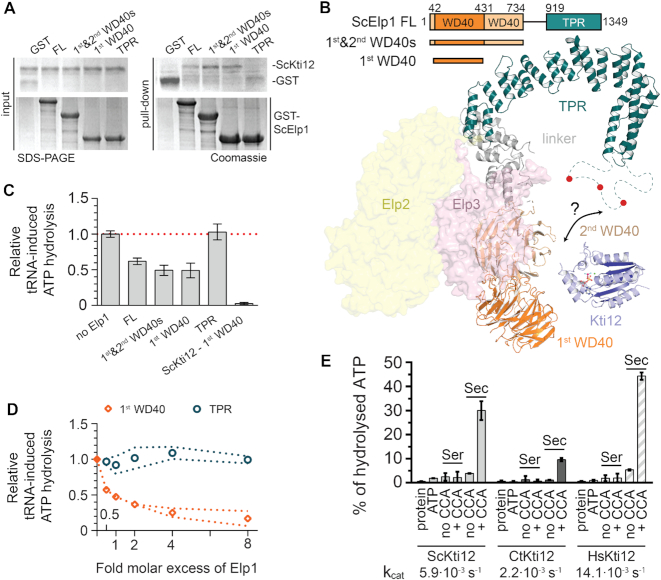
Figure 6.
Functional studies of ScKti12 binding to Elongator. (A) The interaction site on ScElp1 was mapped using domains of ScElp1, namely, first WD40 (aa 42–431), first and second WD40s (aa 1–734) and TRP (aa 919-1349). Interaction was observed only in case of constructs containing the first WD40 domain. Inputs before the pull down are shown on the left and GST-tagged Elp1 proteins are shown below. Gels were stained with Coomassie and respective proteins are labeled next to the gel. (B) A structural model of one lobe of the Elp123 subcomplex of Elongator built using integrative modelling approach. Elp1 consists of two N-terminal WD40 domains (shades of orange), flexible linker region (gray) and a tetratricopeptide motif (TPR; teal) used for dimerization of Elp1 molecules. Unstructured loop region (aa1175–1252) within Elp1 TRP is shown as dashed line. Red circles indicate presence of multiple phosphorylation sites within the loop region. Surfaces of Elp2 (yellow) and Elp3 (pink) proteins are also shown. Kti12NTD (blue) is shown in a vicinity of the identified interaction site. (C) Binding of ScElp1 to the ScKti12 inhibits tRNA-induced hydrolysis. 1 μM ScElp1 protein was added to the reaction mixture containing 1 μM ScKti12. ScKti12 – first WD40 indicates ATP hydrolysis by a fusion protein, in which the first WD40 was linked to the C-ter of ScKti12 using a flexible 5x(GS) linker. Results are normalized to the tRNASec-induced ATP hydrolysis in the absence of Elp1. Means and calculated standard deviations are shown (n = 3). (D) The first WD40 domain of Elp1 inhibits tRNA-dependent ATP hydrolysis in a dose dependent manner. Increasing amounts of first WD40 domain (orange squares) or TPR (tale circles) were added to the ATPase reaction, up to eight times greater molar concentration of Elp1. Results are normalized to the tRNASec-induced ATP hydrolysis in the absence of Elp1. Error corridors represent standard deviations (n = 3). (E) ATP hydrolysis of Kti12 is dependent on maturity of presented tRNA. Kti12 hydrolyses ATP in the presence of tRNASec with a 3′CCA (tRNASec, +CCA). Removal of 3′CCA from tRNASec (tRNASec, no CCA) resulted in almost complete diminishment of inducing properties of tRNASec. ATP hydrolysis was assessed with the malachite green assay. kcat values were calculated based on the ATP hydrolysis after 4 h (data not shown). Tested conditions resulted in following kcat values: kcat (ScKti12) = 5.85×10−3 ± 0.28×10−3 s−1; kcat (CtKti12) = 2.19×10-3 ± 0.05×10−3 s−1; kcat (HsKti12) = 14.05×10−3 ± 0.10·10−3 s−1. PSTK from hyperthermophylic archaea exhibited lower kcat values upon induction by tRNASec, kcat(MjPSTK) = 2.9×10−5 ± 0.3×10−5 s−1 (Sherrer et al., 2008b). Calculated means and standard deviations are shown (n = 3).