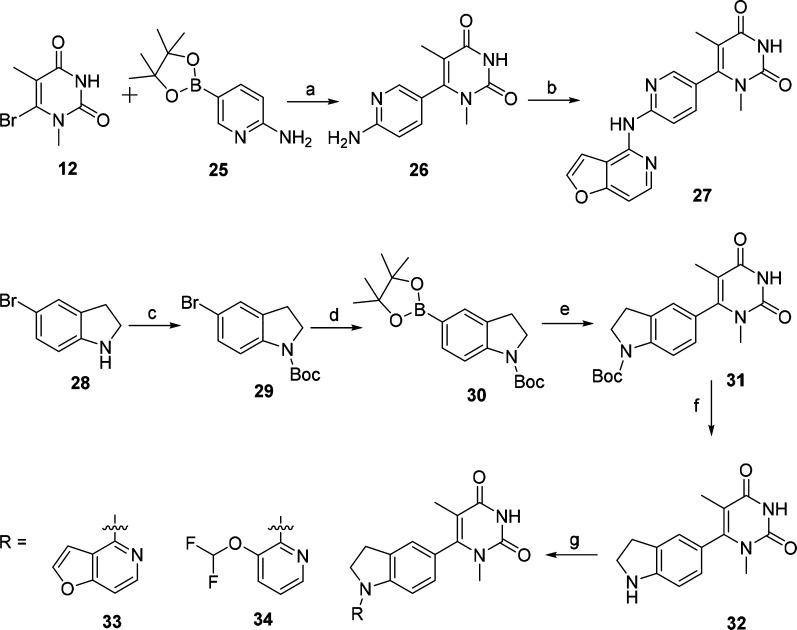
Scheme 2. Synthesis of Compounds 27, 33, and 34.
Reagents and conditions: (a) [1,1′-bis(diphenylphosphino)ferrocene]tdichloropalladium(II), K2CO3, 1,4-dioxane, 100 °C, 12 h, 67%; (b) compound 8, tris(dibenzylideneacetone)dipalladium(0), 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene, K2CO3, 1,4-dioxane, 100 °C, 12 h, 59%; (c) (Boc)2O, Et3N, CH2Cl2, rt, 12 h, 91%; (d) bis(pinacolato)diboron, bis(diphenylphosphino)ferrocene]dichloropalladium(II), KOAc, 1,4-dioxane, 85 °C, 16 h, 81%; (e) 12, Pd(PPh3)4, Na2CO3, 1,4-dioxane, water, 100 °C, 12 h, 77%; (f) (i) CF3COOH, CH2Cl2, rt, 1 h, (ii) K2CO3, MeOH, rt, 12 h, 92%; (g) Pd(OAc)2, 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene, Cs2CO3, 1,4-dioxane, 100 °C, 12 h, 50–73%.