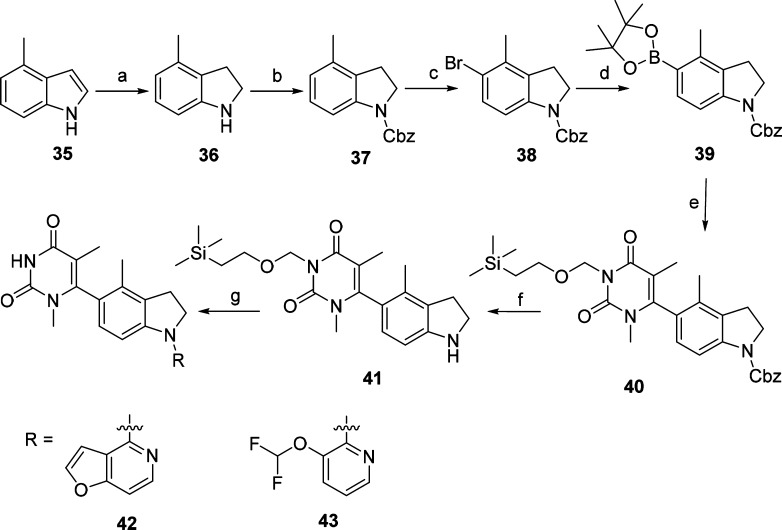
Scheme 3. Synthesis of Compounds 42 and 43.
Reagents and conditions: (a) NaBH3CN, AcOH, 0 °C to rt, 4 h, 68%; (b) CbzCl, Et3N, CH2Cl2, 0 °C to rt, 12 h, 74%; (c) N-bromosuccinimide, CH2Cl2, 0 °C to rt, 12 h, 94%; (d) bis(pinacolato)diboron, [1,1′-bis(diphenylphosphino) ferrocene]dichloropalladium(II), KOAc, 1,4-dioxane, 70 °C, 48 h, 46%; (e) 13, [1,1′-bis(diphenylphosphino)ferrocene] dichloropalladium(II), K2CO3, 1,4-dioxane, 100 °C, 12 h, 38%; (f) Pd/C, H2, rt, 12 h, 96%; (g) (i) Pd(OAc)2, 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene, Cs2CO3, 1,4-dioxane, 100 °C, 12 h, (ii) CF3COOH, CH2Cl2, rt, 1 h, (iii) K2CO3, MeOH, rt, 12 h, 17–24% (three steps).