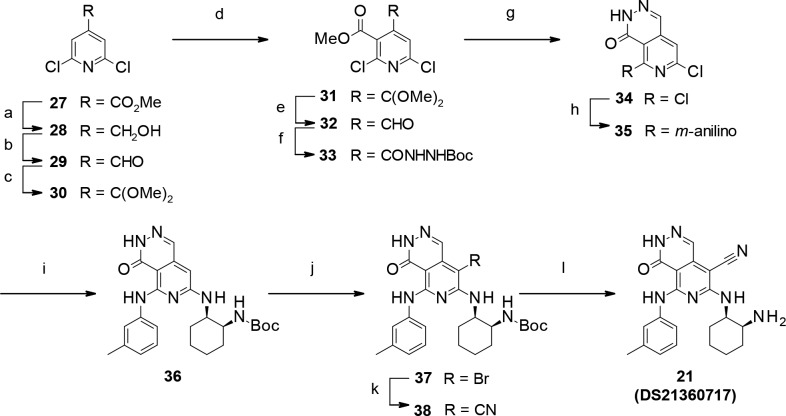
Scheme 2. Synthesis of Pyrido-pyridazinone Derivative 21 (DS21360717).
Reagents: (a) NaBH4, EtOH, reflux, quant.; (b) Dess–Martin periodinane, CH2Cl2, rt, quant.; (c) trimethyl orthoformate, p-TsOH-H2O, MeOH, toluene, reflux, quant.; (d) LDA, chloroformic acid methyl ester, THF, −78 °C, 27%; (e) TFA, CH2Cl2, rt, quant.; (f) carbazic acid tert-butyl ester, 1,4-dioxane, rt, quant.; (g) TFA, CH2Cl2, rt, 99%. (h) 3-methylaniline, NMP, microwave, 160 °C, quant.; (i) tert-butyl [(1S,2R)-2-aminocyclohexyl]carbamate, NMP, microwave 170 °C, 45%; (j) NBS, DMF, rt, 87%; (k) zinc cyanide, tetrakis(triphenylphosphine) palladium(0), DMF, microwave, 120–130 °C, 55%. (l) TFA, CH2Cl2, rt, 87%.