Moxetumomab pasudotox (CAT-8015, HA22) is a recombinant immunotoxin comprised of a variable fragment (Fv) of a murine IgG4 anti-CD22 monoclonal antibody genetically fused to a truncated fragment of Pseudomonas exotoxin A.(Kreitman and Pastan 2011) In a phase I paediatric study in relapsed/refractory acute lymphoblastic leukaemia (ALL), moxetumomab pasudotox, given at a dose of 5-40 μg/kg resulted in a complete remission (CR) rate of 24% and an overall response rate (ORR) of 70%.(Wayne, et al 2011) Here we report a single-centre phase I study of moxetumomab pasudotox in adults with relapsed/refractory B-cell ALL.
Patients aged ≥18 years with previously treated relapsed or refractory B-cell ALL were eligible. Patients were required to have a performance status of ≤2, with an ejection fraction ≥40%, total bilirubin ≤26 μmol/l, liver transaminases ≤2.5 times the upper limit of normal and creatinine clearance ≥50 ml/min. This study was approved by the institutional review board of The University of Texas MD Anderson Cancer Center and registered at ClinicalTrials.gov (NCT01891981). All patients provided informed consent according to the Declaration of Helsinki.
Moxetumomab pasudotox was evaluated over 3 dose levels [30 μg/kg (n=6), 40 μg/kg (n=4), and 50 μg/kg (n=6)] given intravenously over 30 min every other day × 6 doses, with cycle length of 21 days. Patients achieving a response could receive up to 6 cycles or allogeneic stem cell transplantation (AlloSCT). The primary objective was to determine the dose-limiting toxicities (DLTs) and maximum tolerated dose (MTD) of moxetumomab pasudotox. Secondary objectives included the ORR (defined as CR + CR with incomplete count recovery [CRi] + partial remission), event-free survival (EFS) and overall survival (OS).
Between December 2013 and September 2015, 16 adults with relapsed or refractory B-ALL were treated. Baseline characteristics are shown in Table I. The median CD22 expression was 92% (range, 51%−100%). Twelve of 15 patients (80%) tested positive for anti-drug antibodies (ADA) by a screening assay prior to moxetumomab pasudotox administration. There was no correlation between the presence of ADAs and prior monoclonal antibody exposure. The median number of cycles administered was 1 (range, 1-4). Seven patients (44%) received ≥2 cycles of moxetumomab pasudotox. Only one DLT was observed; this patient, who received the 50 μg/kg dose, developed grade 3 haemolytic uraemic syndrome (HUS) after 1 cycle, which resolved with cessation of moxetumomab pasudotox and supportive management.
Table I.
Patient Demographics and Clinical Characteristics (N=16)
| Characteristica | Value |
|---|---|
| Age (years), median (range) | 30 (18-67) |
| Performance status, n (%) | |
| 0 | 2 (13) |
| 1 | 10 (63) |
| 2 | 4 (25) |
| Number of prior treatments, n (%) | |
| 1 | 2 (13) |
| 2 | 5 (31) |
| 3 | 3 (19) |
| ≥4 | 6 (38) |
| Prior inotuzumab ozogamicin, n (%) | 3 (19) |
| Prior stem cell transplant, n (%) | 4 (25) |
| Response to prior treatment, n (%) | |
| Refractory | 10 (63) |
| Early relapse (<6 months) | 2 (13) |
| Late relapse (≥6 months) | 4 (25) |
| Cytogenetics, n (%) | |
| Diploid | 3 (19) |
| High hyperdiploid | 2 (13) |
| Low hypodiploid | 1 (6) |
| t(1;19) | 2 (13) |
| t(9;22) | 1 (6) |
| t(4;11) | 1 (6) |
| Complex | 2 (13) |
| Miscellaneous | 3 (19) |
| Insufficient metaphases | 1 (6) |
| CD22 expression, n (%) | |
| 50-69% | 3 (19) |
| 70-89% | 4 (25) |
| ≥90% | 8 (50) |
| Positiveb | 1 (6) |
Continuous variables are listed as median (range) and categorical variables as n (%)
One patient did not have CD22 expression quantified but was considered “positive” for CD22 by the reviewing pathologist
Grade 3/4 non-haematological adverse events at least possibly related to moxetumomab pasudotox were observed in 7 patients, including: elevation of transaminases (n=3, all transient), weight gain (n=2), capillary leak syndrome (CLS; n=1), HUS (n=1), ascites (n=1), tumour lysis syndrome (n=1), hyperbilirubinaemia (n=1, transient) and hypotension (n=1). The case of CLS was grade 4 and developed during the second cycle of moxetumomab pasudotox at the 30 μg/kg dose level. No patients experienced prolonged myelosuppression attributed to moxetumomab pasudotox. No treatment-related deaths were observed, and the MTD was not reached.
One patient received only 5 doses of moxetumomab pasudotox and was excluded from response analysis. Of the 15 evaluable patients, the ORR was 13% (n=2, 1 CR and 1 CRi). Among 12 patients with pre- and post-CD22 expression levels (all non-responders), there was a non-significant decrease in CD22 expression after moxetumomab pasudotox exposure (mean change ± standard error: −7.3% ± 3.8%; P=0.08). There was no difference in ADA titre levels between responding and non-responding patients, although numbers were limited for this analysis. One patient, who had received 5 prior therapies, including AlloSCT, achieved CR after 1 cycle at the 30 μg/kg dose. This patient received 4 cycles of moxetumomab pasudotox and had a CR duration of 2.5 months. One patient who had received 2 prior therapies achieved CRi after 1 cycle at the 50 μg/kg dose, but developed grade 3 HUS and thus did not receive additional cycles. The CD22 expression of these two responding patients was 98% and 92%, respectively, and both tested positive for ADAs prior to drug exposure. The OS of these two responding patients was 9.0 and 3.9 months, respectively. The median EFS for the entire cohort was 1.1 months, and the median OS was 6.7 months (Figure 1).
Figure 1.
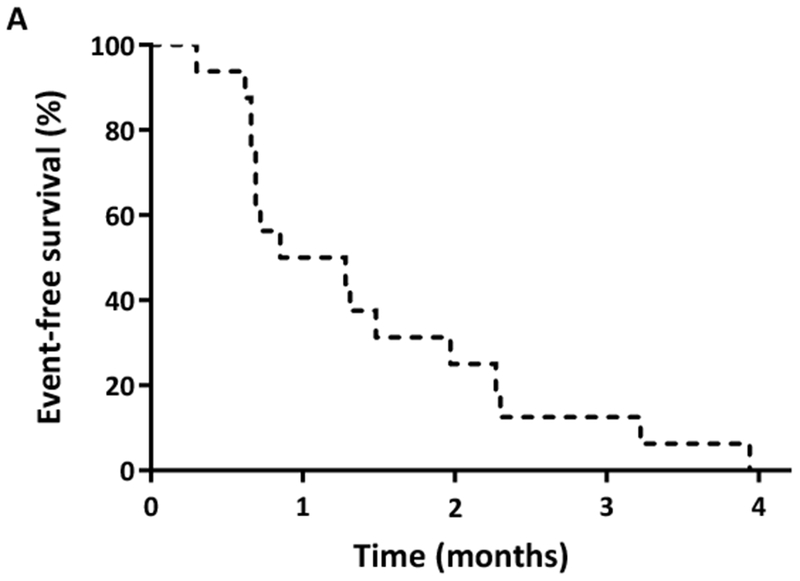
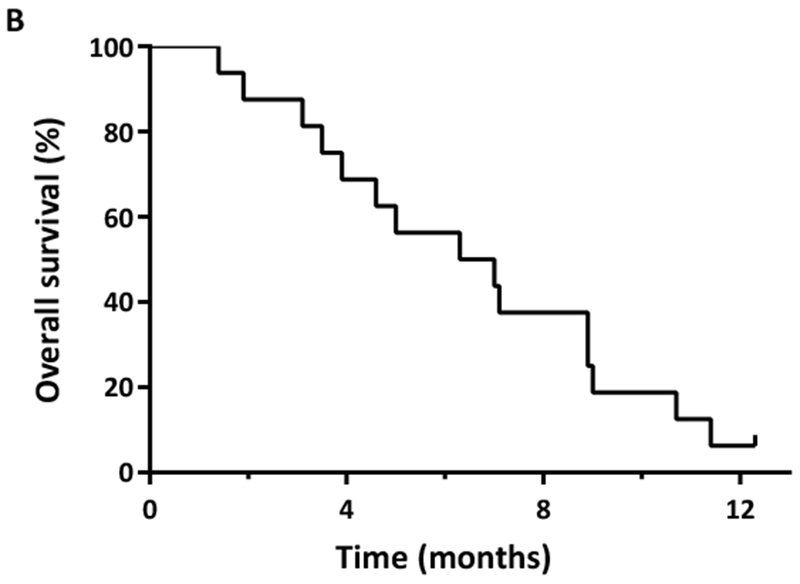
(A) Event-free survival and (B) overall survival of adult patients with relapsed/refractory acute lymphoblastic leukaemia treated with moxetumomab pasudotox.
In this study of adults with heavily pre-treated relapsed/refractory ALL, moxetumomab pasudotox was safe and tolerable up to a dose of 50 μg/kg, resulting in an ORR of 13%. A higher planned dose level of 50 μg/kg × 10 doses was not evaluated because this schedule was not tolerated in a parallel paediatric trial, leading to the early closure of this study by the sponsor. Although the clinical activity of moxetumomab pasudotox was modest at the doses studied, the present study highlights the promise of CD22 as an effective target for anti-leukaemic therapies, including combination approaches with toxins or cytotoxic drugs (Du, et al 2008, Kantarjian, et al 2016, Kreitman and Pastan 2011). Additional modifications of the anti-CD22 immunotoxin may result in improved clinical activity with a larger therapeutic window and less immunogenicity (Onda, et al 2011, Weldon, et al 2009)
As moxetumomab pasudotox contains a bacterial toxin, it was highly immunogenic, as expected. The majority of patients (80%) had ADAs prior to the first administration of moxetumomab pasudotox, probably due to prior exposure to the toxin. These results are higher than the ADA rates reported for adults with hairy cell leukaemia (38%)(Kreitman, et al 2012) and paediatric ALL (14%).(Wayne, et al 2011) While this discrepancy may be related to the small sample size of the present study as well as differences in cut-off levels for positivity, it questions whether the relatively modest clinical activity observed in the current study may be partly due to neutralizing antibodies present at baseline. It is notable however that ADAs were identified prior to moxetumomab pasudotox exposure in both of the responding patients, and therefore the presence of ADAs did not preclude the potential for response.
In conclusion, moxetumomab pasudotox was safe and had modest clinical activity at the doses studied in adults with heavily pre-treated relapsed/refractory ALL. These results reinforce CD22 as a promising therapeutic target in ALL and other haematological malignancies.
Acknowledgements
N.J.S. collected and analysed the data and wrote the manuscript; H.K. designed the study and treated patients; E.J., J.E.C, D.A.T, M.E.R., N.D., Y.A., M.K., P.K., W.G.W. and C.D.D. treated patients; C.B., D.M. and M.A.R. collected and analysed the data; F.R. designed the study, collected and analysed the data, treated patients and wrote the manuscript. All authors approved the final version of the manuscript.
Funding: Supported by Medimmune and the MD Anderson Cancer Center Support Grant CA016672
References
- Du X, Beers R, Fitzgerald DJ & Pastan I (2008) Differential cellular internalization of anti-CD19 and -CD22 immunotoxins results in different cytotoxic activity. Cancer Res, 68, 6300–6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, Gokbuget N, O’Brien S, Wang K, Wang T, Paccagnella ML, Sleight B, Vandendries E & Advani AS (2016) Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med, 375, 740–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitman RJ & Pastan I (2011) Antibody fusion proteins: anti-CD22 recombinant immunotoxin moxetumomab pasudotox. Clin Cancer Res, 17, 6398–6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitman RJ, Tallman MS, Robak T, Coutre S, Wilson WH, Stetler-Stevenson M, Fitzgerald DJ, Lechleider R & Pastan I (2012) Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol, 30, 1822–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda M, Beers R, Xiang L, Lee B, Weldon JE, Kreitman RJ & Pastan I (2011) Recombinant immunotoxin against B-cell malignancies with no immunogenicity in mice by removal of B-cell epitopes. Proc Natl Acad Sci U S A, 108, 5742–5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne AS, Bhojwani D, Silverman LB, Richards K, Stetler-Stevenson M, Shah NN, Jeha S, Pui C-H, Buzoianu M, FitzGerald DJ, Kreitman RJ, Ibrahim R & Pastan I (2011) A Novel Anti-CD22 Immunotoxin, Moxetumomab Pasudotox: Phase I Study in Pediatric Acute Lymphoblastic Leukemia (ALL). Blood, 118, 248–248. [Google Scholar]
- Weldon JE, Xiang L, Chertov O, Margulies I, Kreitman RJ, FitzGerald DJ & Pastan I (2009) A protease-resistant immunotoxin against CD22 with greatly increased activity against CLL and diminished animal toxicity. Blood, 113, 3792–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]


