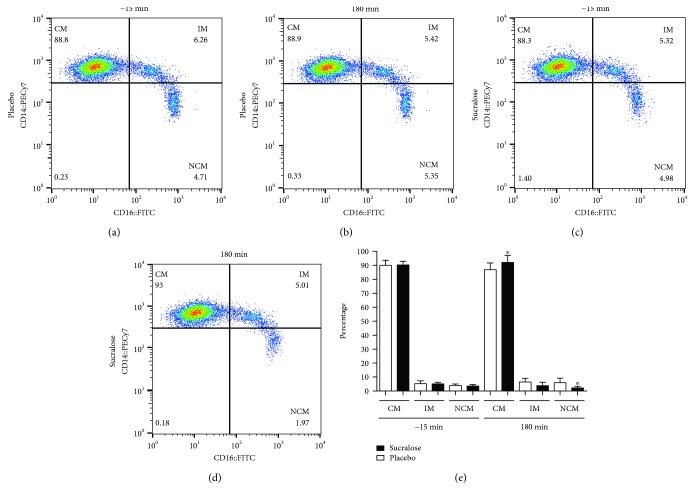
Figure 2.
Percentages of classical, intermediate, and nonclassical monocytes in healthy young adults that received sucralose or placebo at the beginning and at the end of an oral glucose tolerance test. Representative flow cytometry dot plots showing the percentages of classical (CM), intermediate (IM), and nonclassical monocytes (NCM) in the placebo group at the beginning (a) and at the end (b) of the oral glucose tolerance test (OGTT). Representative dot plots showing the percentages of CM, IM, and NCM in the sucralose group at the beginning and at the end of the OGTT can be seen in (c) and (d), respectively. (e) As expected, quantification of monocyte subpopulation percentages showed no differences between placebo and sucralose groups at the beginning of the OGTT (-15 min). At 180 min, the CM percentage significantly increased whereas the NCM percentage decreased in volunteers that received 48 mg sucralose as compared to subjects that received water as placebo. No significant differences were seen in the IM percentage. The placebo group is shown in open bars, whereas the sucralose group can be seen in closed bars. Monocytes were gated on a CD14+CD16+ dot plot to identify monocyte subpopulations as follows: CD14++CD16−, classical monocytes; CD14++CD16+, intermediate monocytes; and CD14+CD16+, nonclassical monocytes. Data are expressed as media ± standard deviation. Significant differences between placebo and sucralose groups were estimated by performing two-tailed, 2-way ANOVA followed by the Bonferroni multiple comparisons test. Significant differences are indicated by asterisks. Differences were considered significant when P < 0.05.