Key Points
Question
Is progression of retinopathy associated with progression of kidney disease in patients with chronic kidney disease?
Findings
In this multicenter cohort study of 1583 patients with chronic kidney disease, fundus photographs obtained at baseline and a mean of 3.5 years later were assessed. Chronic kidney disease worsening was associated with worsening of retinopathy in univariable but not in multivariable analysis.
Meaning
The findings suggest that similar risk factors may be affecting the progression of both retinal and chronic kidney disease.
Abstract
Importance
Associations between retinopathy and kidney disease have been previously described. The association between the progression of retinopathy and concurrent progression of chronic kidney disease is unknown.
Objective
To assess the association between progression of retinopathy and concurrent progression of chronic kidney disease (CKD) among persons with CKD enrolled in a prospective cohort study.
Design, Setting, and Participants
A total of 1936 patients with chronic kidney disease enrolled in the multicenter, prospective Chronic Renal Insufficiency Cohort (CRIC) Study were invited to have 2 nonmydriatic fundus photography sessions separated by a mean (SD) of 3.5 (0.5) years. The study was conducted from May 12, 2006, to June 29, 2011. Data analysis was performed from March 16, 2016, to November 17, 2017.
Main Outcomes and Measures
Fundus photographs obtained at baseline and then at a follow-up at 3.5 years were reviewed by masked graders for presence and severity of retinopathy, and vessel calibers were assessed using standard protocols. The associations of the changes in retinal features with progression of CKD (50% estimated glomerular filtration rate [eGFR] loss or incident end-stage renal disease, and differences in eGFR slope in the same time period) were assessed with univariable and multivariable logistic regression models.
Results
Among 1583 CRIC participants who had baseline fundus photography, had additional follow-up in CRIC, and were at risk for retinopathy progression, 1025 patients (64.8%) had follow-up photography. The odds ratio (OR) for CKD progression associated with worsening of retinopathy in comparison with participants with stable retinopathy was 2.24 (95% CI, 1.28-3.91; P = .005) in univariable analysis among participants with baseline and follow-up photography. In the multivariable analysis, the OR was 1.62 (95% CI, 0.77-3.39; P = .20). The multiple imputation analysis provided similar results.
Conclusions and Relevance
Progression of retinopathy appears to be associated with progression of CKD on univariable analysis but not on multivariable analysis suggesting that similar risk factors may be affecting the progression of both retinal and chronic kidney disease.
This cohort study examines the association between progression of retinopathy and progression of chronic kidney disease in patients with chronic kidney disease.
Introduction
Ocular fundus photography provides noninvasive detection of retinal vascular abnormalities that may reflect vascular abnormalities in other organs, including the kidney. Retinal images may supplement other procedures gathering prognostic information regarding kidney disease.
Chronic kidney disease (CKD) is reported to be associated with other illnesses,1 including eye disease.2,3 The Chronic Renal Insufficiency Cohort (CRIC) Study investigators previously reported an independent, cross-sectional association between retinopathy and kidney dysfunction4 and subsequent progression of CKD5 and cardiovascular disease6 among men and women with CKD who were enrolled in CRIC, a long-term, observational cohort study.7,8 Population-based studies have also shown an association between baseline retinopathy and subsequent progression of CKD.9,10 We extend those observations in the CRIC Study by prospectively evaluating the association between progression of retinal vascular changes and the progression of CKD among CRIC Study participants.7,8
Methods
Study Design
The design and characteristics of the participants of the CRIC study7,8 and the methods used have been reported.4,5,6 A total of 2605 CRIC participants at 6 of the 7 CRIC clinical sites were eligible for fundus photographs. Among them, 1936 CRIC participants (74.3%) had a baseline fundus photography session and 1025 individuals (39.3%) had a second photography session a mean (SD) of 3.5 (0.5) years (range, 2.6-4.9 years) later. Baseline photography was obtained between May 12, 2006, and June 14, 2008, and follow-up photography was obtained between November 12, 2009, and June 29, 2011. Data analysis was performed from March 16, 2016, to November 17, 2017. Our study followed the tenets of the Declaration of Helsinki11 and was approved by the University of Pennsylvania Institutional Review Board. Participants provided written informed consent; they did not receive financial compensation.
Trained and certified nonophthalmic personnel obtained fundus photographs from participants without pharmacologic dilatation. A Canon CR-DGI, Non-Mydriatic Retinal Camera (Canon Inc) was used to obtain 45° digital, color fundus photographs of the macula and optic disc from both eyes at each visit.
Retinopathy and Vessel Caliber Assessment
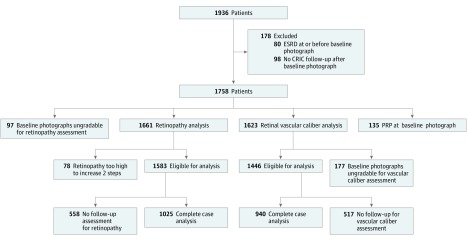
After excluding baseline photographs that were ungradable, fundus pathologic findings, including retinopathy (attributed to diabetes, hypertension, or other) were assessed in 1025 participants and the diameters of the major retinal vessels were measured in 940 participants (Figure). We excluded from vessel caliber analysis 135 participants who had received laser panretinal photocoagulation at baseline in all their gradable eyes, because this treatment greatly reduces vascular diameters.
Figure. Flowchart Describing Study Participants.
CRIC indicates Chronic Renal Insufficiency Cohort study; ESRD, end-stage renal disease.
Because the photograph readers were unaware of the systemic diabetic or hypertensive status of the participants, retinopathy was evaluated without assumption of cause. The Atherosclerosis Risk in Communities fundus photographic12 and the Early Treatment of Diabetic Retinopathy (ETDRS) grading protocols13 were used to grade retinopathy due to diabetes, hypertension, and other conditions. These grading protocols have been previously applied to persons with and without diabetes.14 Digital photographs were evaluated using standard protocols and with standardized photographic field definitions.4,5,6
Retinopathy Grading
Several retinal vascular abnormalities were graded by referring to standard photographs.12,13 From these diverse retinal abnormalities, an overall retinopathy severity score was assigned for each eye based on a modification of the ETDRS diabetic retinopathy scale.5,13,15 There were 10 ordinal levels of the score, ranging from no retinopathy to advanced proliferative retinopathy (PR). Scores for an eye were used to determine the eye’s baseline retinopathy classification (4 categories) as normal, very mild nonproliferative (NPR) retinopathy, moderate and severe NPR, or PR. The eye with more advanced retinopathy was used as the person-level classification at baseline. In the cases when photographs of only 1 eye were available, the score for that eye was used as the person-level category.
To determine progression of retinopathy, the 10-level ordinal scale scores from each of the 2 eyes were combined into a 19-step scale.16 When photographs of only 1 eye were available, we assumed that both eyes had the same level of retinopathy. Progression of retinopathy was defined as an increase of 2 or more steps in the ETDRS person-level score from the first score.16 An increase of 2 or more steps in the ETDRS person-level scores has been accepted in large epidemiologic studies as a clinically meaningful change.16
Measurement of Vascular Diameters
Image processor measurements of vascular arteriolar and venular calibers were performed according to the Atherosclerosis Risk in Communities Study protocol,12 using interactive vessel analysis software developed at the University of Wisconsin. The diameters of up to 6 arterioles and venules were averaged,5,12 providing separate average arteriolar and venular changes. A difference of 10 μm or more in vessel diameter between baseline and follow-up was considered a meaningful change based on reproducibility of measurement.4,5
CKD Outcomes
Outcomes related to CKD were collected for the period between initial photography and follow-up photography. We did not include any renal data after the second photography session. The renal outcomes were (1) 50% estimated glomerular filtration rate (eGFR) loss; (2) incident end-stage renal disease (ESRD) requiring initiation of chronic dialysis therapy or kidney transplantation, with ascertainment of ESRD in the CRIC study supplemented by linkage with the US Renal Data System; and (3) the slope of eGFR over time, where eGFR was calculated using a CRIC internal GFR-estimating equation derived from iothalamate 125 clearance testing.17,18 In this study, 2 measures of progression of CKD were used: a composite of ESRD or 50% decline in eGFR and the slope of eGFR between baseline and follow-up.
Statistical Analysis
We compared baseline characteristics of participants with vs without follow-up photographs using χ2 tests for comparisons of proportions and 2-tailed, unpaired t tests for means. Participants who had advanced retinopathy at the time of the baseline photography session and, therefore, could not worsen by 2 or more steps, were excluded from analysis. We also excluded participants who had had ESRD at the baseline photography session (Figure). We evaluated the association of worsening of retinopathy between initial and follow-up photography with development of ESRD or 50% eGFR decrease by using univariable logistic regression models, and multivariable models adjusted for age, race/ethnicity, body mass index, baseline smoking status, diabetes, systolic blood pressure, initial eGFR, initial retinopathy level, and clinical site. We also assessed the association of 2 or more-step worsening in retinal level with eGFR slope between the initial and follow-up fundus photographs using a linear mixed-effect model unadjusted and adjusted for the covariates specified above. We first performed these analyses using only patients who had both initial and follow-up photographs (2-imaging session cohort).
Owing to the large number of patients (558 [35.2%]) who did not have follow-up photographs, either because of death or loss of follow-up, we performed multiple imputation19 of missing data for the change in the person-level retinopathy score. Among those without follow-up photographs but at least 1 subsequent eGFR measurement, we first selected the time interval between the initial photograph and follow-up photograph by random sampling of time intervals from patients who had both initial and follow-up photographs. If the selected time was after the last eGFR measurement, the time of that eGFR measurement was used as the imputed time; otherwise, the selected time was used as the imputed time. Next, we imputed whether 2 or more-step retinal level worsening had occurred by using a logistic regression model that included as covariates previously identified predictors of change in retinopathy4,5 measured at the time of the initial photographs and the imputed length of follow-up. We added the imputed data to the data from the cohort patients with photographs at both times (imputation-added cohort) and performed the regression analyses, as described above. We repeated these steps 50 times and combined the regression analysis results using proc mianalyze in SAS. A 2-tailed P value less than .05 was considered statistically significant. All statistical analyses were performed in SAS, version 9.4 (SAS Institute Inc).
Results
Among 1936 participants with initial photographs, we excluded from the analysis 80 participants who had ESRD before the initial photography, 98 participants who had no CRIC follow-up after initial photography, 97 who had initial photographs that were deemed ungradable, and 78 who had a retinopathy level that could not worsen by 2 or more steps (Figure). Among the remaining 1583 participants, 1025 individuals (64.8%) had follow-up photography and 558 individuals (35.2%) did not. Of the 558 participants who did not have follow-up photography, 47 individuals (8.4%) died before the beginning of the follow-up imaging period and 59 participants (10.6%) died during the follow-up period. The interval between baseline and follow-up photography sessions was a mean (SD) of 3.5 (0.5) years (range, 2.6-4.9 years).
In comparison with the 1025 participants who had follow-up photography, the 558 participants who did not were older (mean [SD], 61.2 [10.7] vs 59.3 [10.5] years; P < .001) and less often non-Hispanic white (252 [45.2%] vs 531 [51.8%]; P = .02), had higher systolic blood pressure (128.6 [22.1] vs 123.7 [20.1] mm Hg; P < .001), and more had diabetes (283 [50.7%] vs 397 [38.7%]; P < .001), advanced retinopathy level (34 [6.1%] vs 42 [4.1%]; P = .03), and worse initial eGFR (42.3 [17.9] vs 47.6 [18.2] mL/min/1.73 m2; P < .001) (Table 1).
Table 1. Comparison of Baseline Characteristics Between Participants by Follow-up Photograph Status.
| Initial Photography Session Characteristics | Total (N = 1583) | Follow-up Photograph Status | P Valuea | |
|---|---|---|---|---|
| Without (n = 558) | With (n = 1025) | |||
| Retinal level, No. (%) | ||||
| No NPR | 1170 (73.9) | 403 (72.2) | 767 (74.8) | .03 |
| Mild NPR | 124 (7.8) | 38 (6.8) | 86 (8.4) | |
| NPR | 213 (13.5) | 83 (14.9) | 130 (12.7) | |
| PR | 76 (4.8) | 34 (6.1) | 42 (4.1) | |
| Vessel diameter, mean (SD), μm | ||||
| Arteriole | 149 (14.4) | 149 (15.0) | 149 (14.1) | .90 |
| Venule | 219 (23.5) | 220 (23.9) | 219 (23.3) | .80 |
| eGFR, mean (SD), mL/min/1.73 m2 | 45.7 (18.3) | 42.3 (17.9) | 47.6 (18.2) | <.001 |
| 24-h Urine protein, mean (SD), g/24 h | 0.51 (1.21) | 0.59 (1.33) | 0.47 (1.14) | .07 |
| Age, mean (SD), y | 60.0 (10.6) | 61.2 (10.7) | 59.3 (10.5) | <.001 |
| Race, No. (%) | ||||
| Non-Hispanic white | 783 (49.5) | 252 (45.2) | 531 (51.8) | .02 |
| Non-Hispanic black | 662 (41.8) | 260 (46.6) | 402 (39.2) | |
| Hispanic | 64 (4.0) | 18 (3.2) | 46 (4.5) | |
| Other | 74 (4.7) | 28 (5.0) | 46 (4.5) | |
| Sex, No. (%) | ||||
| Male | 856 (54.1) | 296 (53.0) | 560 (54.6) | .54 |
| Female | 727 (45.9) | 262 (47.0) | 465 (45.4) | |
| Diabetes, No. (%) | ||||
| No | 903 (57.0) | 275 (49.3) | 628 (61.3) | <.001 |
| Yes | 680 (43.0) | 283 (50.7) | 397 (38.7) | |
| Smoking, No. (%) | ||||
| Nonsmoker | 727 (45.9) | 248 (44.4) | 479 (46.7) | .35 |
| Former | 671 (42.4) | 241 (43.2) | 430 (42.0) | |
| Current | 185 (11.7) | 69 (12.4) | 116 (11.3) | |
| BMI, No. (%)b | ||||
| Normal (<25) | 274 (17.4) | 91 (16.5) | 183 (17.9) | .45 |
| Overweight (25-<30) | 470 (29.9) | 165 (29.9) | 305 (29.9) | |
| Obese (≥30) | 828 (52.7) | 296 (53.6) | 532 (52.2) | |
| Systolic BP, mean (SD), mm Hg | 125.4 (20.9) | 128.6 (22.1) | 123.7 (20.1) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; eGFR, estimated glomerular filtration rate; NPR, nonproliferative retinopathy; PR, proliferative retinopathy.
P values are trend tests for retinal level, smoking, and BMI; χ2 tests for other categorical variables; and t tests for continuous variables.
Data missing for some patients.
Among 1025 patients who had baseline and follow-up photographs, 930 individuals (90.7%) had no retinopathy progression, 26 individuals (2.5%) had 2-step worsening, 32 patients (3.1%) had 3-step worsening, and 37 patients (3.6%) had more than 3-step worsening, with a total of 95 participants (9.3%) having 2-step or more worsening. Worsening of 2 steps or more retinal level from initial assessment occurred in 30 of 628 participants (4.8%) without diabetes, and 65 of 397 participants (16.4%) with diabetes. Among 930 participants who had no progression of retinopathy, 88 patients (9.5%) developed ESRD or 50% eGFR decrease. Of 95 participants with progression of retinopathy, 18 (19%) developed ESRD or 50% eGFR decrease (Table 2). Of the 106 participants who progressed to ESRD or 50% eGFR decrease, 18 patients (17.0%) had retinopathy progression. Only 18 of 1025 participants (1.8%) had progression of both retinopathy and CKD, and 832 patients (81.2%) had no progression of either factor. There were 44 participants (4.3%) with regression of retinopathy by 2 or more steps: 5 with PDR (11.4%), 38 with NPR (86.4%), and 1 with mild NPR (2.3%).
Table 2. Association of 2-Step or More Retinopathy Worsening With ESRD or 50% eGFR Decrease Between Initial and Follow-up Photography.
| Group | Retinal Worsening | No. (%) | ESRD or 50% eGFR Decrease, No. (%) | Univariable Analysis | Multivariable Analysisa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-Image Sessions Cohort | Imputation Added Cohort | 2-Image Sessions Cohort | Imputation Added Cohort | |||||||||||
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |||||||
| All (N = 1025) | No | 930 (90.7) | 88 (9.5) | 1 [Reference] | .005 | 1 [Reference] | .02 | 1 [Reference] | .20 | 1 [Reference] | .48 | |||
| Yes | 95 (9.3) | 18 (18.9) | 2.24 (1.28-3.91) | 1.83 (1.09-3.06) | 1.62 (0.77-3.39) | 1.29 (0.63-2.66) | ||||||||
| Diabetes (n = 397) | No | 332 83.6) | 55 (16.6) | 1 [Reference] | .50 | 1 [Reference] | .51 | 1 [Reference] | .19 | 1 [Reference] | .71 | |||
| Yes | 65 (16.4) | 13 (20.0) | 1.26 (0.64-2.47) | 1.23 (0.66-2.29) | 1.92 (0.72-5.13) | 1.18 (0.49-2.86) | ||||||||
| No diabetes (n = 628) | No | 598 (95.2) | 33 (5.5) | 1 [Reference] | .02 | 1 [Reference] | .06 | 1 [Reference] | .33 | 1 [Reference] | .44 | |||
| Yes | 30 (4.8) | 5 (16.7) | 3.42 (1.23-9.52) | 2.50 (0.97-6.45) | 1.98 (0.50-7.88) | 1.71 (0.44-6.70) | ||||||||
| P value for interaction with diabetes | .11 | .22 | .17 | .56 | ||||||||||
Abbreviations: eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; OR, odds ratio.
Adjusted for eGFR, retinal level, age, race/ethnicity, body mass index, smoking, diabetes, systolic blood pressure, and clinical site at initial session.
For the association between 2 steps or more retinopathy worsening and development of a 50% eGFR loss or incident ESRD, the univariable analysis showed an odds ratio (OR) of 2.24 (95% CI, 1.28-3.9; P = .005) for study participants with initial and follow-up photography (Table 2). When participants without follow-up photography were included in the univariable analysis using multiple imputation (n = 1583), the OR was 1.83 (95% CI, 1.09-3.06; P = .02). In multivariable analysis, however, the associations were no longer statistically significant; the OR was 1.62 (95% CI, 0.77-3.39; P = .20) for the complete data analysis and 1.29 (95% CI, 0.63-2.66; P = .48) for the imputation analysis.
We further investigated these associations in participants with and without diabetes at baseline. We found no significant interaction between diabetes and differences in the association between risk of retinopathy progression and development of ESRD or 50% eGFR decrease (Table 2). However, among participants without diabetes, those who had 2 or more steps of retinopathy progression (n = 30) had a higher risk of progression of kidney disease on univariable analysis (OR, 3.42; 95% CI, 1.23-9.52; P = .02) than those who did not have 2 or more steps of retinopathy progression (Table 2). This association was no longer significant after adjusting for baseline covariates (OR, 1.98; 95% CI, 0.50-7.88; P = .33) (Table 2). This association was not observed in participants with diabetes (OR, 1.26; 95% CI, 0.64-2.47; P = .50). Similar results were found for the analysis including imputed data.
For the association between 2-step progression of retinopathy and eGFR slope (millimeters per minute per 1.73 m2), the univariable analysis for participants with baseline and follow-up photographs showed that participants with a 2-step progression of retinopathy had a greater decline in eGFR compared with those without such retinal disease progression (mean slope difference, −0.91; 95% CI, −1.56 to −0.25; P = .007) (Table 3). For the imputed data cohort, the univariable analysis showed a mean slope difference of −0.67 (95% CI, −1.35 to 0.02; P = .06). Multivariable analysis showed a slope difference of −0.36 (95% CI, −1.03 to 0.31; P = .30) for the complete cohort and −0.22 (95% CI, −0.95 to 0.51; P = .55) for the imputed cohort. We did not detect an interaction between diabetes and differences in the association between risk of retinopathy progression and eGFR slope (Table 3).
Table 3. Association of 2-Step or More Retinopathy Worsening With eGFR Slope Between Initial and Follow-up Photography.
| Group | Analysis Cohort | No.a | eGFR Slope, Mean (SE), mL/min/1.73 m2/y | Difference in eGFR Slope Between Retinopathy Worsening vs Not Worsening, mL/min/1.73m2/y | ||||
|---|---|---|---|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysisb | |||||||
| Mean (95% CI) | P Value | Mean (95% CI) | P Value | |||||
| All | 2-Image sessions | 999 | −0.20 (0.10) | −0.91 (−1.56 to −0.25) | .007 | −0.36 (−1.03 to 0.31) | .30 | |
| Imputation added | 1517c | −0.25 (0.09) | −0.67 (−1.35 to 0.02) | .06 | −0.22 (−0.95 to 0.51) | .55 | ||
| Diabetes | 2-Image sessions | 378 | −0.79 (0.16) | −0.54 (−1.40 to 0.32) | .22 | −0.13 (−1.05 to 0.80) | .79 | |
| Imputation added | 640c | −0.67 (0.14) | −0.37 (−1.27 to 0.53) | .42 | −0.13 (−1.11 to 0.86) | .80 | ||
| No diabetes | 2-Image sessions | 621 | 0.17 (0.12) | −0.72 (−1.81 to 0.38) | .20 | −0.40 (−1.46 to 0.67) | .47 | |
| Imputation added | 877c | 0.07 (0.11) | −0.60 (−1.73 to 0.53) | .30 | −0.31 (−1.45 to 0.84) | .60 | ||
| P value for interaction with diabetes | 2-Image sessions | .97 | .90 | |||||
| Imputation added | .99 | .77 | ||||||
Abbreviation: eGFR, estimated glomerular filtration rate.
Twenty-six patients without sufficient eGFR data were excluded from eGFR slope analysis.
Adjusted for eGFR, retinal level, age, race/ethnicity, body mass index, smoking, diabetes, systolic blood pressure, and clinical site at initial session.
The mean number of patients in the imputed analysis varied depending on the imputed follow-up time.
Regarding vessel diameter assessment, of 1936 participants, 80 individuals (4.1%) had ESRD prior to the initial photography session and 98 patients (5.1%) had no CRIC follow-up after the initial photography session. We excluded 135 participants who had panretinal laser photocoagulation in all of their gradable eyes at baseline, 177 who had ungradable photographs for vascular caliber assessment, and 517 who had no follow-up photography (Figure).
Among study participants who had initial and follow-up photographs, changes in arteriole diameters were not significantly associated with risk of ESRD or 50% eGFR decrease in univariable analysis (increase ≥10: OR, 1.59 [95% CI, 0.75-3.38]; decrease ≥10: OR, 1.54 [95% CI, 0.96-2.49]; P = .15) or in multivariable analysis (increase ≥10: OR, 0.83 [95% CI, 0.29-2.37]; decrease ≥10: OR, 1.62 [95% CI, 0.86-3.04]; P = .27; P for interaction with diabetes = .14). Changes in venule diameters were also not significantly associated with risk of ESRD or 50% eGFR decrease in univariable analysis (increase ≥10: OR, 1.46 [95% CI, 0.80-2.65]; decrease ≥10: OR, 1.74 [95% CI, 1.07-2.82]; P = .07) or in multivariable analysis (increase ≥10: OR, 1.30 [95% CI 0.61-2.76]; decrease ≥10: OR, 2.01 [95% CI, 1.07-3.75]; P = .09; P for interaction with diabetes = .22) (Table 4). These findings were unchanged using multiple imputation analysis.
Table 4. Association of Change in Retinal Vascular Diameters With ESRD or 50% eGFR Decrease Between Initial and Follow-up Photography in All Participants (n = 957).
| Artery Brancha | Change in Retinal Vascular Diameter, μm | No. | ESRD or 50% eGFR Decrease, No. (%) | Univariable Analysis | Multivariable Analysisb | ||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Valuec | OR (95% CI) | P Valuec | ||||
| Arteriole | ≤−10 | 235 | 29 (12.3) | 1.54 (0.96 to 2.49) | .15 (.24) | 1.62 (0.86 to 3.04) | .27 (.048) |
| >−10 to <10 | 634 | 53 (8.4) | 1 [Reference] | 1 [Reference] | |||
| ≥10 | 71 | 9 (12.7) | 1.59 (0.75 to 3.38) | 0.83 (0.29 to 2.37) | |||
| Venule | ≤−10 | 267 | 34 (12.7) | 1.74 (1.07 to 2.82) | .07 (.09) | 2.01 (1.07 to 3.75) | .09 (.24) |
| >−10 to <10 | 517 | 40 (7.7) | 1 [Reference] | 1 [Reference] | |||
| ≥10 | 156 | 17 (10.9) | 1.46 (0.80 to 2.65) | 1.30 (0.61 to 2.76) | |||
Abbreviations: eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; OR, odds ratio.
Only the 2 image sessions cohort was used for these analyses.
Adjusted for initial session eGFR, initial session respective retinal vascular diameter, age, race, body mass index, smoking, diabetes, systolic blood pressure, and clinical site.
P value not in parentheses represents the comparison of ESRD or 50% eGFR decrease across 3 levels of change in retinal vascular diameter. P value in parentheses represents the association of retinal vascular diameter as a continuous measure with ESRD or halving of eGFR.
We also investigated the association between retinal vasculature diameter changes and eGFR slopes but did not find any statistically significant associations on univariable or multivariable analysis. These findings were unchanged after multiple imputation analysis.
Discussion
Our results suggest an association between progression of retinopathy and progression of CKD in unadjusted analyses. This finding is not surprising since previous studies have shown that retinopathy is associated with nephropathy.3,4,5,6,9,10,20,21 Our present results go further in that they show evidence of a concurrent progression of these conditions although most of the cases demonstrating progression of retinopathy did not have CKD progression and vice versa. In our study, only 18 participants (1.8%) had progression of both retinopathy and CKD and 832 individuals (81.2%) had no progression of either disease.
When adjusted in a multivariate analysis for baseline characteristics described in Table 1, these associations between retinopathy progression and CKD progression were not statistically significant, suggesting that data on the progression of retinopathy may not add information regarding CKD progression to that provided by these other traditional risk factors.
The presence of an association between progression of retinal and renal abnormalities in univariable analysis, but not in multivariable analysis, could be because traditional risk factors used in the multivariable analysis may influence the progression of both eye and kidney disease in a similar fashion, suggesting a common pathogenetic pathway. Alternatively, this difference between univariable and multivariable findings could be due to other factors.
Previous studies have looked into the cross-sectional association between baseline retinopathy and CKD, as well as retinopathy as a risk factor for subsequent progression of CKD.3,4,5,6,9,10,20,21 To our knowledge, our study is the first to report on a large-scale, long-term, prospective, multicenter investigation of the association between progression of retinopathy and progression of kidney disease among individuals with established CKD.
Because 558 of the 1583 participants in our study did not have follow-up photographs and they tended to have more advanced retinopathy and CKD, we performed additional analyses using multiple imputation of missing data. In general, the results with and without imputation for missing data did not differ much. The univariable analysis with multiple imputation showed significant associations between progression of retinopathy and progression of kidney disease, while the multivariable analysis did not show a significant association.
Although we did not find a significant interaction between diabetes and the association between the changes in retinopathy and CKD outcomes, in the unadjusted analysis we detected a statistically significant association between progression of retinopathy and CKD in participants without diabetes (OR, 3.42; P = .02) (Table 2). This association was not significant in participants with diabetes (OR, 1.26; P = .50). Possibly, the pathologic changes in diabetes may affect the changes occurring in kidney disease. Additional studies are needed to replicate these findings.
Previous articles from the CRIC Study group found associations between baseline retinopathy and incident CKD4 and future renal function decline.5 The study presented herein centered on the associations between change in time in retinopathy and CKD within a relatively short mean period of 3.5 years. Differences between these previous article and the present one may be the result of the relatively short period of follow-up.
Previous studies have shown associations between retinal vascular diameters and CKD,20,21,22 although these studies did not look at associations between progression of CKD and progression of retinopathy. Our study did not show any such associations. This finding could be because of our relatively small sample size or the short follow-up time.
Limitations
Our study has several limitations. The lack of follow-up photographs in 35.2% of the participants may have masked stronger associations. To address this limitation we performed multiple imputation analyses, which did not show major differences from the analysis of the complete data set. In addition, our follow-up time was relatively short and the statistical power was somewhat low. Another weakness of our study is that we do not have detailed information on ocular and kidney treatments, which may weaken our conclusions. The cohort of this study was not large and the analysis of the outcomes of the many different systemic treatments is not feasible. Because the last follow-up photograph was taken on June 29, 2011, none of our patients received antivascular endothelial growth factor treatment, which was not available at that time.
Our study shows that 9.3% of all participants and 16.4% of participants with diabetes had retinopathy progression of 2 or more steps. In participants with diabetes, this number is a bit lower than values shown in a previous population study reported in 1989.23,24 Differences in the study populations and improvements in diabetes treatment since the 1980s may explain these discrepancies. Because a 2-step retinopathy progression is associated with future progression of retinal disease in patients with diabetes,16 these patients should have a careful follow-up.
Conclusions
The results of our study suggest an association between retinopathy progression and concurrent CKD progression in univariable analysis. The fact that this association is not significant after adjustment for known risk factors for both disease processes suggests that progression of CKD and retinopathy may be mediated by similar mechanisms. In addition, our results suggest substantial prevalence and progression of retinopathy in participants with CKD, justifying vigilance for monitoring eye disease in patients with CKD.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. . Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038-2047. doi: 10.1001/jama.298.17.2038 [DOI] [PubMed] [Google Scholar]
- 2.Wong WW, Wong TT, Cheung CY, Sabanayagam C. Kidney and eye diseases: common risk factors, etiological mechanisms, and pathways. Kidney Int. 2014;85(6):1290-1302. doi: 10.1038/ki.2013.491 [DOI] [PubMed] [Google Scholar]
- 3.Grunwald JE, Alexander J, Maguire M, et al. ; The CRIC Study Group . Prevalence of ocular fundus pathology in subjects with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:867-873. doi: 10.2215/CJN.08271109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grunwald JE, Alexander J, Ying GS, et al. ; CRIC Study Group . Retinopathy and chronic kidney disease in the Chronic Renal Insufficiency Cohort (CRIC) study. Arch Ophthalmol. 2012;130(9):1136-1144. doi: 10.1001/archophthalmol.2012.1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grunwald JE, Pistilli M, Ying GY, et al. ; Chronic Renal Insufficiency Cohort Study Investigators . Retinopathy and progression of CKD: the CRIC Study. Clin J Am Soc Nephrol. 2014;9:1217-1224. doi: 10.2215/CJN.11761113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grunwald JE, Pistilli M, Ying GS, et al. ; CRIC Study Investigators . Retinopathy and the risk of cardiovascular disease in patients with chronic kidney disease (from the Chronic Renal Insufficiency Cohort study). Am J Cardiol. 2015;116(10):1527-1533. doi: 10.1016/j.amjcard.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman HI, Appel LJ, Chertow GM, et al. ; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators . The Chronic Renal Insufficiency Cohort (CRIC) Study: design and methods. J Am Soc Nephrol. 2003;14(7)(suppl 2):S148-S153. doi: 10.1097/01.ASN.0000070149.78399.CE [DOI] [PubMed] [Google Scholar]
- 8.Lash JP, Go AS, Appel LJ, et al. ; Chronic Renal Insufficiency Cohort (CRIC) Study Group . Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302-1311. doi: 10.2215/CJN.00070109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong TY, Coresh J, Klein R, et al. . Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. J Am Soc Nephrol. 2004;15(9):2469-2476. doi: 10.1097/01.ASN.0000136133.28194.E4 [DOI] [PubMed] [Google Scholar]
- 10.Edwards MS, Wilson DB, Craven TE, et al. . Associations between retinal microvascular abnormalities and declining renal function in the elderly population: the Cardiovascular Health Study. Am J Kidney Dis. 2005;46(2):214-224. doi: 10.1053/j.ajkd.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 11.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 12.Hubbard LD, Brothers RJ, King WN, et al. ; Atherosclerosis Risk in Communities Study Group . Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106(12):2269-2280. doi: 10.1016/S0161-6420(99)90525-0 [DOI] [PubMed] [Google Scholar]
- 13.Early Treatment Diabetic Retinopathy Study Research Group Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification: ETDRS report number 10. Ophthalmology. 1991;98(5)(suppl):786-806. doi: 10.1016/S0161-6420(13)38012-9 [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Klein BE, Moss SE, Wong TY. The relationship of retinopathy in persons without diabetes to the 15-year incidence of diabetes and hypertension: Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2006;104:98-107. [PMC free article] [PubMed] [Google Scholar]
- 15.Wong TY, Klein R, Islam FM, et al. . Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141(3):446-455. doi: 10.1016/j.ajo.2005.08.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein R, Klein BE, Moss SE. How many steps of progression of diabetic retinopathy are meaningful? the Wisconsin epidemiologic study of diabetic retinopathy. Arch Ophthalmol. 2001;119(4):547-553. doi: 10.1001/archopht.119.4.547 [DOI] [PubMed] [Google Scholar]
- 17.Dobre M, Yang W, Chen J, et al. ; CRIC Investigators . Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: a report from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2013;62(4):670-678. doi: 10.1053/j.ajkd.2013.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson AH, Yang W, Hsu CY, et al. ; CRIC Study Investigators . Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60(2):250-261. doi: 10.1053/j.ajkd.2012.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, Inc; 1987. doi: 10.1002/9780470316696 [DOI] [Google Scholar]
- 20.Sabanayagam C, Shankar A, Klein BEK, et al. . Bidirectional association of retinal vessel diameters and estimated GFR decline: the Beaver Dam CKD Study. Am J Kidney Dis. 2011;57:682-691. doi: 10.1053/j.ajkd.2010.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ooi QL, Tow FK, Deva R, et al. . The microvasculature in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(8):1872-1878. doi: 10.2215/CJN.10291110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabanayagam C, Shankar A, Koh D, et al. . Retinal microvascular caliber and chronic kidney disease in an Asian population. Am J Epidemiol. 2009;169(5):625-632. doi: 10.1093/aje/kwn367 [DOI] [PubMed] [Google Scholar]
- 23.Klein R, Klein BEK, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy—IX: four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1989;107(2):237-243. doi: 10.1001/archopht.1989.01070010243030 [DOI] [PubMed] [Google Scholar]
- 24.Klein R, Klein BEK, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy—X: four-year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Arch Ophthalmol. 1989;107(2):244-249. doi: 10.1001/archopht.1989.01070010250031 [DOI] [PubMed] [Google Scholar]