Figure 2.
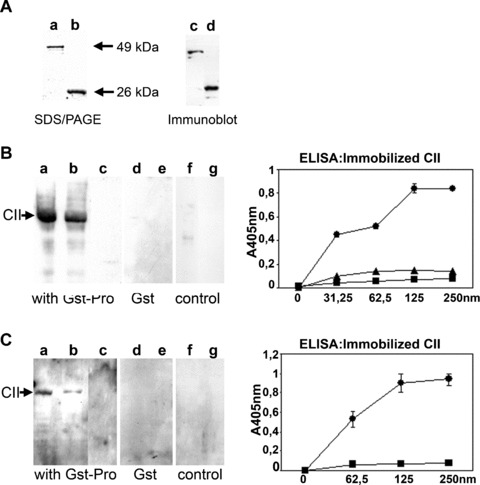
Binding of Pro‐Gst fusion protein to native and denatured collagen type II (CII). (A) SDS/PAGE of the purified Pro‐Gst at 49 kD and Gst‐tag at 26 kD (lanes a, b) and the corresponding immunoblot using anti‐Gst antibodies (lanes c, d). (B, C) Binding of Pro‐Gst to CII and BSA was performed using Far Western Technique. After electrophoretic separation on a 10% polyacrylamide gel, CII (5, 2.5 μg, lanes a, b; 5 μg, lanes d, f) and BSA (5 μg, lanes c, e, g) were transferred to a nitrocellulose filter, incubated with Pro‐Gst and detected with anti‐Gst (B) and anti‐prodomain antibodies (C). Incubation with the Gst‐tag alone is shown in (B, C; lanes d, e). Controls were performed without addition of Pro‐Gst, but using the detection antibodies (B, C; lanes f, g). Using ELISA technique, soluble Pro‐Gst (0–250 nM) was incubated on immobilized native CII (•) or denatured CII (▪) and detected with anti‐Gst (B) or anti‐prodomain antibodies (C). B, ▴ incubation of the Gst‐tag with native CII. Mean values and standard deviation from triplicates are shown.
