Figure 7.
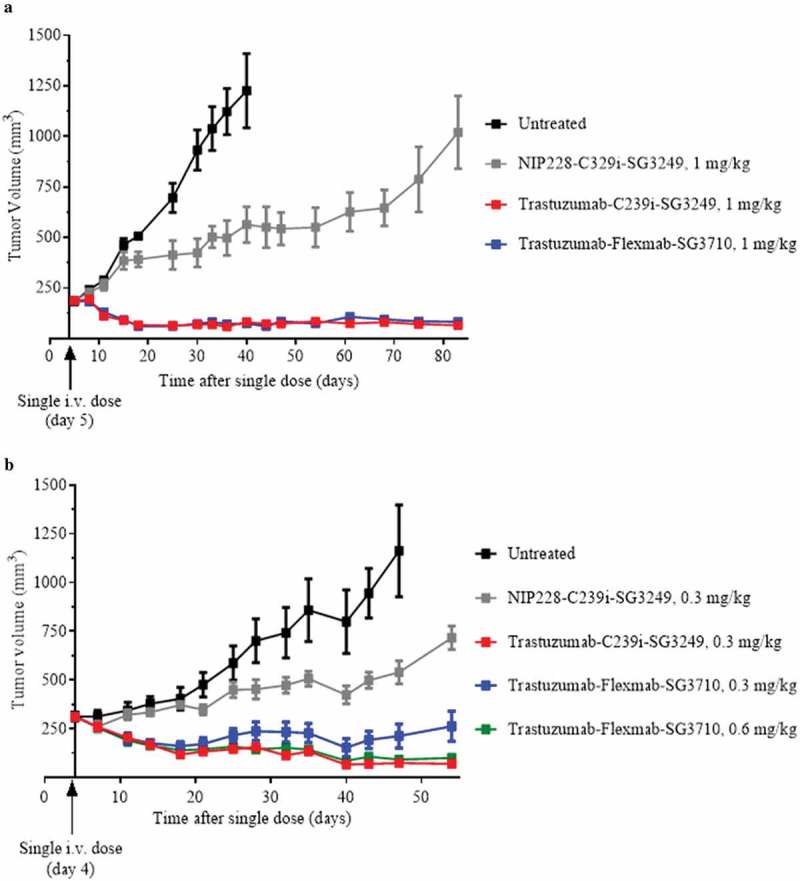
In vivo efficacy results in female athymic mice bearing NCI-N87 HER2-positive subcutaneous xenografts. (a) Results for 1 mg/kg dose of NIP228-C239i-SG3249 (grey), trastuzumab-C239i-SG3249 (red) and trastuzumab-Flexmab-SG3710 (blue). Five mice per group were dosed intravenously five days after their tumors reached a volume of 200 mm3. (b) In this efficacy study, the intravenous dose of NIP228-C239i-SG3249 (grey) and trastuzumab-C239i-SG3249 (red) were 0.3 mg/kg, while trastuzumab-Flexmab-SG3710 was dosed at 0.3 mg/kg (blue) and 0.6 mg/kg (green). The mice were treated on day four after tumors reached a volume of 200 mm3. Untreated mice were used as negative controls (black).
