Figure 4.
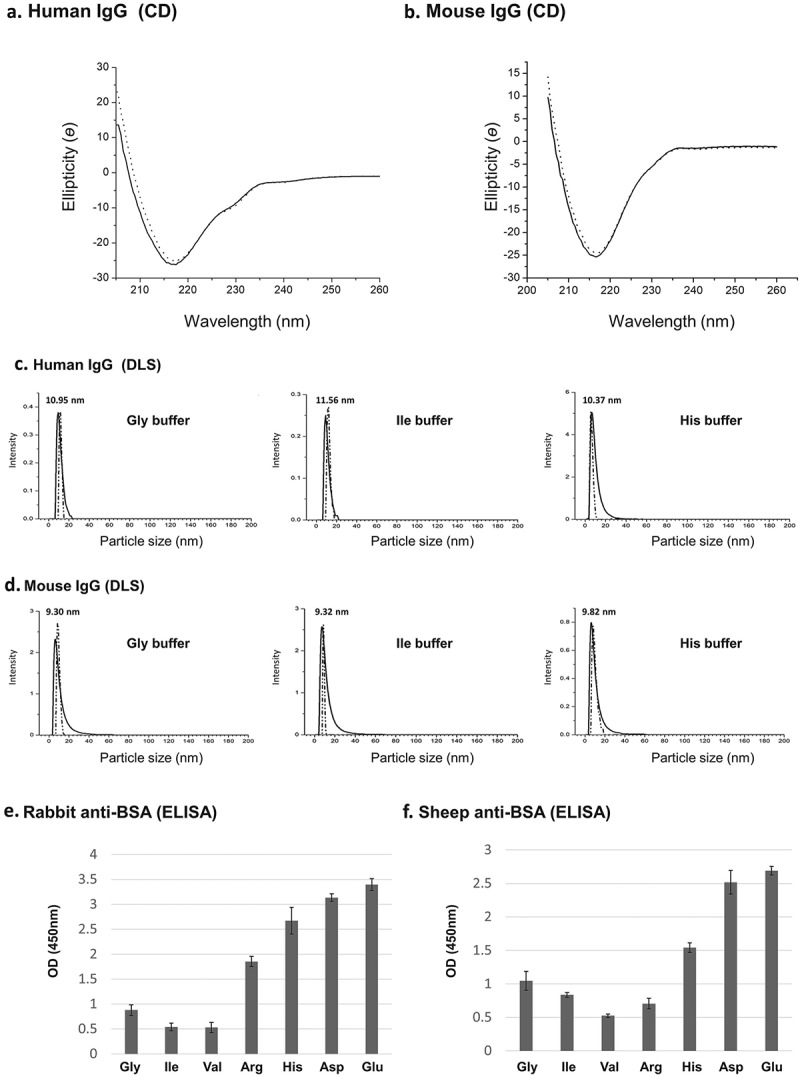
(a-b) CD spectra. Human and mouse IgGs were subjected to purification with Tween-20 aggregates and extracted with Gly buffer at pH 3.8. CD spectra of control IgGs (i.e., untreated IgGs – solid line) and IgGs that were subjected to purification (dotted line) are shown. (c-d) Dynamic light scattering (DLS). Human and mouse IgGs were purified as described in A-B and extracted with indicated buffers at pH 3.8. Control untreated IgGs – solid line, purified IgGs – dotted line. (e-f) ELISA analysis of extracted IgGs. Polyclonal anti-BSA IgGs originating from rabbit (naked) or sheep (biotinylated) were subjected to the micelle-based purification method and extracted at 32 °C (5 min.) from Tween-20 aggregates with the amino acid buffers indicated (50 mM) at pH 3.8. The ability of the purified Ab to bind target epitopes on BSA was determined by ELISA assays as described in the Material and Methods section. The data presented are the results of at least 12 independent experiments.
