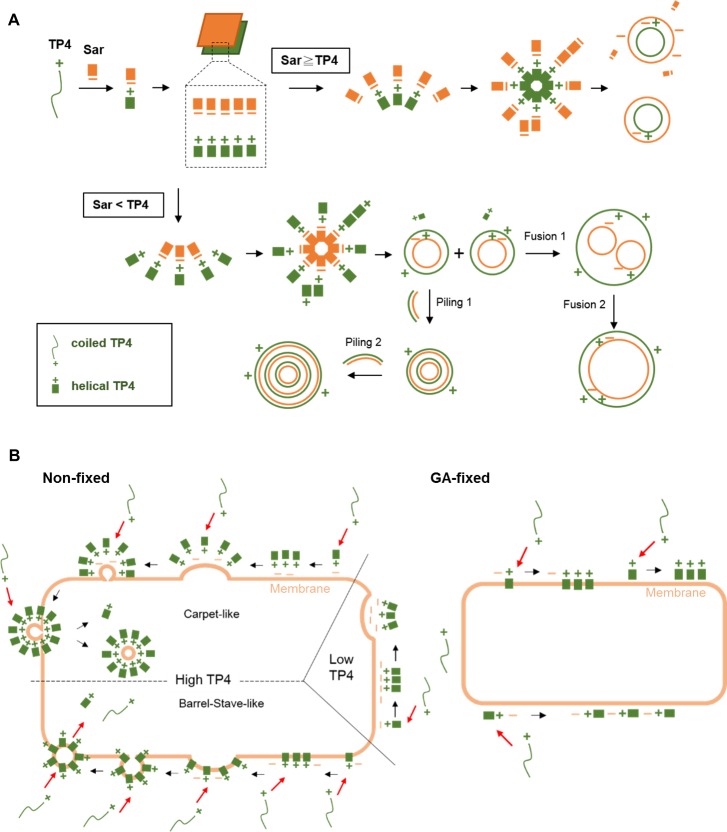
Fig 11. Proposed model of AMP assembly and entry into E. coli.
(A). Assembly of cationic TP4 peptides into vesicles by sarkosyl (Sar). The TP4 peptides (green) are driven into α-helical and amphipathic structure by sarkosyl (orange). The TP4 peptides and sarkosyl are aligned into individual plane through hydrophobic interactions, then assembled into TP4/sarkosyl bilayers by electrostatic interaction. Various kinds of vesicle may be formed from these bilayers depending on the concentration of TP4 and sarkosyl employed. High ratios of sarkosyl/TP4 favor the formation of soluble and anionic vesicles, while lower ratios render the vesicles hydrophobic and insoluble in aqueous solution. In other words, high concentrations of TP4 peptides favor the fusion of small vesicles into large vesicles by hydrophobic interaction. (B) Entry of TP4 peptides into E. coli. In non-fixed cells, TP4 peptides are likely to cluster and form a bilayer with the bacterial membrane by electrostatic interaction, then form inward curvatures with bacterial membrane at low TP4 concentrations and form outward vesicles at high TP4 concentrations in a carpet-like mode. Alternatively, the hydrophobic core of clustered TP4 peptides may insert into the bacterial membrane in barrel-stave-like mode which resembles the enlargement of TP4/sarkosyl vesicles caused by sarkosyl or TFE addition. Since the formation of TP4/sarkosyl vesicle is reversible, TP4 peptides can be taken from medium and released into cytosol through the assembly and insertion on bacterial membrane. GA-fixed bacteria are not permeable to SYTOX Green and the binding of TP4 peptides to the bacteria membrane is suggested to be mediated through hydrophobic interaction.