Abstract
Background
Ready‐to‐use lipid‐based nutrient supplements (LNS) are a highly nutrient‐dense supplement, which could be a good source of macro‐ and micronutrients for pregnant women who need to supplement their nutrient intake.
Objectives
To assess the effects of LNS for maternal, birth and infant outcomes in pregnant women. Secondary objectives were to explore the most appropriate composition, frequency and duration of LNS administration.
Search methods
In May 2018, we searched CENTRAL, MEDLINE, Embase, 22 other databases and two trials registers for any published and ongoing studies. We also checked the reference lists of included studies and relevant reviews, and we contacted the authors of included studies and other experts in the field to identify any studies we may have missed, including any unpublished studies.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs that compared LNS given in pregnancy to no intervention, placebo, iron folic acid (IFA), multiple micronutrients (MMN) or nutritional counselling.
Data collection and analysis
We used standard Cochrane procedures.
Main results
We included four studies in 8018 pregnant women. All four studies took place in stable community settings in low‐ and middle‐income countries: Bangladesh, Burkina Faso, Ghana and Malawi. None were in emergency settings. The oldest trial was published in 2009. Of the four included studies, one compared LNS to IFA, one compared LNS to MMN, and two compared LNS to both IFA and MMN.
We considered the included studies to be of medium to high quality, and we rated the quality of the evidence as moderate using the GRADE approach.
LNS versus IFA
Maternal outcomes: there was no difference between the LNS and IFA groups as regards maternal gestational weight gain per week (standard mean difference (SMD) 0.46, 95% confidence interval (CI) −0.44 to 1.36; 2 studies, 3539 participants). One study (536 participants) showed a two‐fold increase in the prevalence of maternal anaemia in the LNS group compared to the IFA group, but no difference between the groups as regards adverse effects. There was no difference between the two groups for maternal mortality (risk ratio (RR) 0.53, 95% CI 0.12 to 2.41; 3 studies, 5628 participants).
Birth and infant outcomes: there was no difference between the LNS and IFA groups for low birth weight (LBW) (RR 0.87, 95% CI 0.72 to 1.05; 3 studies, 4826 participants), though newborns in the LNS group had a slightly higher mean birth weight (mean difference (MD) 53.28 g, 95% CI 28.22 to 78.33; 3 studies, 5077 participants) and birth length (cm) (MD 0.24 cm, 95% CI 0.11 to 0.36; 3 studies, 4986 participants). There was a reduction in the proportion of infants who were small for gestational age (SGA) (RR 0.94, 95% CI 0.89 to 0.99; 3 studies, 4823 participants) and had newborn stunting (RR 0.82, 95% CI 0.71 to 0.94; 2 studies, 4166 participants) in the LNS group, but no difference between the LNS and IFA groups for preterm delivery (RR 0.94, 95% CI 0.80 to 1.11; 4 studies, 4924 participants), stillbirth (RR 1.14; 95% CI 0.52 to 2.48; 3 studies, 5575 participants) or neonatal death (RR 0.96, 95% CI 0.14 to 6.51). The current evidence for child developmental outcomes is not sufficient to draw any firm conclusions.
LNS versus MMN
Maternal outcomes: one study (662 participants) showed no difference between the LNS and MMN groups as regards gestational weight gain per week or adverse effects. Another study (557 participants) showed an increased risk of maternal anaemia in the LNS group compared to the MMN group.
Birth and infant outcomes: there was no difference between the LNS and MMN groups for LBW (RR 0.92, 95% CI 0.74 to 1.14; 3 studies, 2404 participants), birth weight (MD 23.67 g, 95% CI −10.53 to 57.86; 3 studies, 2573 participants), birth length (MD 0.20 cm, 95% CI −0.02 to 0.42; 3 studies, 2567 participants), SGA (RR 0.95, 95% CI 0.84 to 1.07; 3 studies, 2393 participants), preterm delivery (RR 1.15, 95% CI 0.93 to 1.42; 3 studies, 2630 participants), head circumference z score (MD 0.10, 95% CI −0.01 to 0.21; 2 studies, 1549 participants) or neonatal death (RR 0.88, 95% CI 0.36 to 2.15; 1 study, 1175 participants).
Authors' conclusions
Findings from this review suggest that LNS supplementation has a slight, positive effect on weight at birth, length at birth, SGA and newborn stunting compared to IFA. LNS and MMN were comparable for all maternal, birth and infant outcomes. Both IFA and MMN were better at reducing maternal anaemia when compared to LNS. We did not find any trials for LNS given to pregnant women in emergency settings.
Readers should interpret the beneficial findings of the review with caution since the evidence comes from a small number of trials, with one‐large scale study (conducted in community settings in Bangladesh) driving most of the impact. In addition, effect sizes are too small to propose any concrete recommendation for practice.
Plain language summary
Effects of lipid‐based nutrient supplements (LNS) for women during pregnancy
Review question
Is giving lipid‐based nutrient supplements (LNS) to women during pregnancy good for mothers and their babies?
Background
Women's nutritional status before and during pregnancy plays a key role in fetal growth and development. It is important to address maternal undernourishment in order to improve maternal and child health. LNS aim to deliver nutrients to pregnant women and other vulnerable people, thereby providing a range of vitamins and minerals coupled with energy, protein and essential fatty acids.
Study characteristics
We found four studies with 8018 pregnant women. The oldest study was published in 2009. All studies took place in developing countries: three were in Africa (one apiece in Ghana, Malawi, and Burkina Faso), and one was in Asia (Bangladesh). All studies took place in a stable community setting; none were conducted in emergency settings. Of the four included studies, one compared LNS to iron folic acid (IFA), one compared LNS to multiple micronutrients (MMN), and two compared LNS to both IFA and MMN.
Key results
This review suggests that there may be a slight benefit of LNS on babies who are born small, and on newborn weight and length, when compared to IFA. LNS did not seem to give any additional benefit to women and newborns compared to MMN, and both IFA and MMN were better at reducing maternal anaemia than LNS. We did not find any studies on LNS for pregnant women in emergency settings.
Quality of evidence
Overall, the evidence is of moderate quality.
Currentness of evidence
The evidence is current to May 2018.
Summary of findings
Background
Description of the condition
The nutritional status of women prior to and during pregnancy plays a key role in fetal growth and development, and women's energy and protein requirements significantly increase during pregnancy (FAO/WHO/UNU 2004). Maternal undernutrition is still prevalent, especially in low‐ and middle‐ income countries (LMICs), with approximately 20% of women in Asia and 10% women in Africa having a low body mass index (BMI; less than 18.5 kg/m² in adult women) (Black 2013). Apart from low BMI, deficiencies of micronutrients, including iron, folate, calcium, and vitamins A and D are also prevalent in LMICs. In 2011 the global prevalence of anaemia among pregnant women was estimated to be 38.2% (WHO 2015). At least half of this anaemia burden is assumed to be due to iron deficiency, with the rest due to other conditions, including folate, vitamin B12 or vitamin A deficiencies; chronic inflammation; parasitic infections; and inherited disorders (Black 2013). Calcium and vitamin D deficiencies are also a major public health problem worldwide in all age groups; however, most countries are still lacking reliable data, particularly population representative data, with limited information on infants, children, adolescents and pregnant women (Palacios 2014). Globally, the prevalence of night blindness in pregnant women is estimated to be around 8%, affecting around 10 million women, with an estimated 15.3% of pregnant women worldwide having low serum retinol levels (Black 2013). Estimates suggest that 28.5% of the world's population, or 1.9 billion individuals, are iodine deficient (Black 2013). Additionally, undernutrition and micronutrient deficiencies increase the risk of infections, and in turn lead to further undernutrition (Black 2013).
Maternal undernutrition causes maternal and child morbidity and mortality, also contributing to low birth weight (LBW) and small‐for‐gestational‐age (SGA) births, which can lead to stunting, wasting and micronutrient deficiencies in children (Black 2013). The placenta forms the interface between maternal‐fetal circulations, which is critical for fetal nutrition and oxygenation (Belkacemi 2010), while the placental supply of nutrients to the fetus depends on its size, morphology, blood supply, and transporter abundance. An optimal maternal nutrient supply has a critical role in placental‐fetal growth and development, while a suboptimum maternal nutrition supply during pregnancy results in intrauterine growth restriction (IUGR) and newborns with LBW (Belkacemi 2010). Maternal iron deficiency anaemia has been strongly associated with adverse birth outcomes, including LBW and increased perinatal mortality, while maternal zinc and iodine deficiencies have been suggested as risk factors for adverse fetal and infant growth (Black 2013). LBW, defined by the World Health Organization (WHO) as weight‐at‐birth of less than 2500 g (5.5 lb), continues to be a significant and global public health problem. Overall, about 15% to 20% of all births worldwide are LBW, representing more than 20 million births a year (WHO 2014). LBW is not only a major predictor of mortality and morbidity in infants and children, but recent studies have found that it also increases the risk for non‐communicable diseases, such as diabetes and cardiovascular disease, in later life (Larroque 2001; Risnes 2011). Nutriton is one of the factors influencing cognitive development, and there is some evidence that these nutritional deficiencies can impair child cognition (Shenkin 2004), as well as pose adverse health outcomes in adulthood (Harder 2007). Literature suggests that there is a connection between improved nutrition and optimal brain function since nutrients provide building blocks in cell proliferation, DNA synthesis, neurotransmitter and hormone metabolism, and they are important constituents of enzyme systems in the brain (Bhatnagar 2001; De Souza 2011; Lozoff 2006; Zeisel 2006; Zimmermann 2011). Brain development is faster in the early years of life, making children more vulnerable to maternal and early life dietary deficiencies (Nyaradi 2013).
Addressing undernutrition by achieving appropriate energy intakes (in the form macronutrients) and ensuring that the intakes of specific nutrients (like vitamins and minerals) are adequate to meet maternal and fetal need, is of the utmost importance for improving maternal and child health outcomes (Imdad 2011). Early preventive measures could address general deprivation and inequity, leading to substantial and long‐term improvements in outcomes. Implementation of nutrition interventions and provision of delivery platforms for hard‐to‐reach populations is also crucial (Black 2013). Disruption and displacement of populations in emergency situations (including conflicts and natural disaster) pose an additional threat to the existing situation of undernutrition. Statistics suggest that women and children represent over three‐quarters of the estimated 80 million people in need of humanitarian assistance, and many countries with high maternal, newborn and child mortality rates are affected by humanitarian emergencies (UNICEF 2014).
Description of the intervention
Various interventions are recommended (or have been implemented) to improve maternal nutrition, including education, food provision and micronutrient supplements (iron, folic acid, multiple micronutrients) as well as other indirect interventions such as agricultural and financial interventions (Bhutta 2013). One of the nutritional interventions advocated to improve undernutrition in pregnant women is lipid‐based nutrient supplements (LNS). Adequate consumption of long‐chain, omega‐3 polyunsaturated fatty acids in the diet of pregnant women is essential, particularly the most biologically active forms (docosahexaenoic acid and eicosapentaenoic acid) (Coletta 2010), as these fatty acids support fetal growth, especially the brain and eyes, and deficiency may be associated with visual deficit and suboptimal behavioural development. However, there is not enough evidence to support the routine use of marine oil or other prostaglandin precursor supplements during pregnancy to reduce the risk of pre‐eclampsia, preterm birth, LBW or SGA (Makrides 2006).
LNS are a family of products designed to deliver nutrients to vulnerable people. There is no standard composition of LNS; however, most of the energy is supplied from fats. Three main LNS products are currently used in maternal and child nutrition: ready to‐use therapeutic foods (RUTF) or large‐quantity (LQ) LNS; ready‐to‐use supplementary foods (RUSF) or medium‐quantity (MQ) LNS; and LNS for home fortification or small‐quantity (SQ) LNS (Arimond 2015). RUTF or LQ‐LNS are designed for treatment of severe acute malnutrition, provide almost all energy requirements, and are given in large daily doses (Diop 2003); RUSF or MQ‐LNS are designed for treatment of moderate acute malnutrition and provide 50% to 100% of energy needed; while SQ‐LNS products are designed to prevent undernutrition and promote growth and development through home fortification of the local diet, and they provide less than 50% of the energy needed (Arimond 2015).
LNS provide a range of vitamins and minerals, but unlike most other micronutrient supplements they also provide energy, protein and essential fatty acids (Chaparro 2010). They are considered 'lipid‐based' because most of the energy provided by these products is from lipids (fats). There is no recommended composition for LNS, so existing projects have used different composition mixes. LNS recipes can include a variety of ingredients, but they typically include vegetable fat, peanut or groundnut paste, milk powder and sugar; other ingredients include whey, soy protein isolate, and sesame, cashew and chickpea paste (iLiNS Project 2016). Various commercial and locally available products are being used as LNS; however, researchers are exploring alternative recipes and formulations in efforts to develop affordable and culturally acceptable products for a range of settings. Similar products combining vegetable oil, groundnut paste, milk, sugar and micronutrients are being used as RUTF for managing both moderate and severe acute malnutrition in infants and children (WHO 2012; WHO 2013). Some studies have evaluated the feasibility and acceptability of LNS, suggesting that it is acceptable to infants as well as pregnant and lactating women (Adu‐Afarwuah 2011). Corn soy blends are different from LNS, as these are fortified blended foods (FBF) used as complementary foods or as supplementary foods for pregnant women.
LNS can be used as point‐of‐use food fortification or can be consumed directly during pregnancy as a source of energy, as protein and micronutrients in public health programmes, and as an intervention to improve birth weight and other pregnancy outcomes in areas where maternal undernutrition is prevalent (Arimond 2015; iLiNS Project 2016). These are usually given at a daily dose of less than 120 kcal/day. The doses and formulations of LNS can be modified according to the needs of the specific target group, and to date, there is no widely accepted, standard formulation for women during pregnancy. Some of the advantages of LNS are that they are ready‐to‐eat (no cooking required) and can be stored for as long as 18 to 24 months even in hot climates (Phuka 2008). This makes LNS especially useful in emergency settings where safe water and hygiene are common issues.
How the intervention might work
Ready‐to‐use lipid‐based nutrients could be a good source of macro‐ and micronutrients in a highly nutrient‐dense supplement, and they could be used as a dietary supplement to address the nutrient requirements of undernourished populations of pregnant women. The supplement composition can be tailored to meet the nutritional requirements of the target population. Cost is an important consideration but should be weighed against the effectiveness in maintaining and improving nutritional outcomes (Chaparro 2010).
Multiple studies have evaluated the impact of LNS when given to pregnant women and children in LMICs. The use of LNS has been associated with improved nutritional status among pregnant women and thereby improved growth and development outcomes among infants and children (Arimond 2015; Iannotti 2014; Thakwalakwa 2010), mainly by focusing on promotion of fetal growth during pregnancy through maternal supplementation. Cord leptin, produced by fetal adipocytes and placenta, has been positively associated with birth size and fetal fat mass (Clapp 1998; Forhead 2009; Lepercq 2001). Some authors hypothesise that LNS increases birth size, possibly through a change in the endocrine regulation of fetal development, and it is associated with higher cord blood leptin in primigravidae and women from the highest BMI tertile (Huybregts 2013). One study suggested that the initial effects of LNS are not sustained during infancy based on the hypothesis that fetuses adapted to better nutritional conditions in utero, which could have made them more sensitive to suboptimal nutritional and environmental factors during the postnatal period (Lanou 2014). One study from Malawi suggested that LNS did not influence the occurrence of maternal infections with Plasmodium falciparum parasitaemia, trichomoniasis or vaginal candidiasis, or of urinary tract infection (Nkhoma 2017). Other studies have suggested that LNS are palatable and acceptable to women in LMIC settings (Adu‐Afarwuah 2011; Mridha 2012; Mridha 2016a), although there were variances to adherence within the population (Harding 2014), as beneficiaries tended to make their own adaptations in terms of how much and how often to consume (Harding 2014). A study evaluating home delivery of LNS products in rural Malawi suggested that the cost of procurement, storage and weekly home delivery of LNS was largely comparable to other product delivery mechanisms currently undertaken in the public sector; however, the study also suggested that the expected health and other benefits associated with each proposed intervention strategy should be compared to the costs to set priorities (Vosti 2015).
Why it is important to do this review
LNS are currently being used in programmes targeting pregnant women in LMICs like Ghana, Malawi and Burkina Faso, with the expectation of improving birth outcomes and reducing LBW (Schofield 2009; WHO 2007). Current studies have shown mixed effects of this intervention using varying compositions, doses, frequencies and comparison groups between studies. Various types of fortified foods are currently in use, including FBF, complementary food supplements and multiple micronutrient powders (MNPs). The type and amount of nutrient varies according to the various products and formulations (Schofield 2009). This review will assess the effects and safety of LNS for women during pregnancy on maternal, birth and infant outcomes, as there is currently no systematic evaluation on this topic. The findings could inform policy and would be highly relevant for countries or areas that have a high burden of undernutrition or those facing emergency situations. We will attempt to assess the appropriate composition, frequency and duration of this intervention through various subgroup analyses. In addition, we will carry out a subgroup analysis on whether the pregnant women were identified and the LNS distributed through a facility or in a community. We are also developing a companion review to assess the effectiveness of preventive LNS plus complementary foods for health, nutrition and developmental outcomes in non‐hospitalised infants and children aged 6 to 23 months (Das in press). Together, these reviews will inform policy decisions on the effectiveness and safety of LNS compared to other interventions, and to assess which delivery platforms are effective.
Multiple micronutrient supplements and powders are currently not recommended for pregnant women (WHO 2016), but we will compare them to the provision of LNS, given the assumption that other micronutrients contained in the micronutrient powders could have an impact on pregnancy, birth and infant developmental outcomes; for example, zinc on preterm births (Ota 2015a). We will also compare LNS to antenatal nutrition education, since nutrition education conducted during the antenatal period with the aim of increasing energy and protein intake appears to be effective in reducing the risk of preterm birth and LBW, increasing head circumference at birth, increasing birth weight among undernourished women, and increasing protein intake (Ota 2015b).
Objectives
To assess the effects of LNS for maternal, birth and infant outcomes in pregnant women. Secondary objectives were to explore the most appropriate composition, frequency and duration of LNS administration.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs (i.e. trials that use methods of assignment such as alternation, or assignment based on date of birth or case record number; Lefebvre 2011).
Types of participants
Women with singleton pregnancy of any age and parity, living in stable or emergency settings.
Types of interventions
Interventions involving the provision of LNS for point‐of‐use food fortification or direct consumption, irrespective of dose, frequency and duration. We included any LNS regardless of its content. Specifically, we assessed the evidence for the following comparisons.
LNS versus no intervention or placebo.
LNS versus IFA ‐ in the form of tablets or capsules. We included studies comparing LNS versus IFA and reported differences in micronutrients between groups, as described by trial authors.
LNS versus oral multiple micronutrient (MMN) supplements ‐ in the form of tablets or capsules. We included studies comparing LNS versus MMN and reported differences in micronutrients between groups, as described by trial authors.
LNS versus multiple micronutrient powders (MNPs) ‐ to be sprinkled over food. We included studies comparing LNS versus MMP and reported differences in micronutrients between groups, as described by trial authors.
LNS versus nutrition counselling ‐ WHO strategy on nutrition counselling during pregnancy focuses primarily on: promoting a healthy diet by increasing the diversity and amount of foods consumed; promoting adequate weight gain through sufficient and balanced protein and energy intake; and promoting consistent and continued use of micronutrient supplements, food supplements or fortified foods.
We included only trials that combined the provision of LNS with other cointerventions, or other approaches, provided the same cointerventions were given to both the intervention and the comparison groups.
This review excluded comparisons to FBF; these foods are given in larger quantities (more calories and nutrients) and so are difficult to compare with LNS, which are given in much smaller quantities.
Types of outcome measures
Primary outcomes
Maternal outcomes
Maternal anthropometric status (weight, BMI, gestational weight gain)
Maternal anaemia at term or near term (haemoglobin (Hb) less than 110 g/L)
Maternal mortality
Adverse effects (any); for example, allergic reactions as diagnosed by clinical assessment (atopic dermatitis, urticaria, oedema (oral), ophthalmic pruritus, allergic rhinitis, asthma, anaphylaxis) or gastrointestinal effects
Birth and infant outcomes
Low birth weight (birth weight less than 2500 g)
Weight at birth (in g)
Length at birth (in cm)
Small‐for‐gestational age (as defined by trial authors)
Preterm births (births before 37 weeks of gestation)
Development outcomes (milestones, as defined by trial authors)
Secondary outcomes
Maternal outcomes
Maternal Hb at term or near term (in g/L at 34 weeks gestation or more)
Duration of gestation
Maternal satisfaction with LNS (as defined by trial authors)
Maternal adherence or compliance with LNS (as defined by study authors)
Birth and infant outcomes
Miscarriage and stillbirths (as defined by trial authors)
Head circumference (in cm)
Mid upper arm circumference (MUAC; in cm)
Stunting at any time within the first six months (length‐for‐age is more than two standard deviations (SD) below the WHO Child Growth Standards median)
Wasting at any time within the first six months (weight‐for‐length is more than two SDs below the WHO Child Growth Standards median)
Underweight at any time within the first six months (weight‐for‐age is more than two SDs below the WHO Child Growth Standards median)
Neonatal death (death occurring between birth and 28 days of life)
Infant mortality (death occurring in the first year of life)
Search methods for identification of studies
Electronic searches
We searched the sources listed below in March 2017, and again in May 2018, using the strategies in Appendix 1. We did not apply language or date restrictions.
International databases
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 4) in the Cochrane Library, and which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register (searched 29 May 2018).
MEDLINE Ovid (1946 to May Week 3 2018).
MEDLINE In‐Process and Other Non‐Indexed Citations Ovid (25 May 2018).
MEDLINE Epub ahead of print Ovid (25 May 2018).
Embase Ovid (1974 to 2018 Week 22).
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1937 to 29 May 2018).
Science Citation Index Web of Science (SCI; 1970 to 28 May 2018).
Social Sciences Citation Index Web of Science (SSCI; 1970 to 28 May 2018).
Conference Proceedings Citation Index ‐ Science Web of Science (CPCI‐S; 1970 to 28 May 2018).
Conference Proceedings Citation Index ‐ Social Science & Humanities Web of Science (CPCI‐SS&H; 1970 to 28 May 2018).
Cochrane Database of Systematic Reviews (CDSR; 2018, Issue 5) part of the Cochrane Library (searched 29 May 2018 ).
Database of Abstracts of Reviews of Effect (DARE; 2015, Issue 2. Final issue) part of the Cochrane Library (searched 9 March 2017).
Epistemonikos (epistemonikos.org; searched 29 May 2018).
POPLINE (www.popline.org; searched 29 May 2018).
ClinicalTrials.gov (clinicaltrials.gov; searched 4 June 2018).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; who.int/trialsearch; searched 4 June 2018).
Regional databases
IBECS (Índice Bibliográfico Español en Ciencias de la Salud; ibecs.isciii.es; searched 21 May 2018).
SciElo (Scientific Electronic Library Online; www.scielo.br; searched 21 May 2018).
AIM Africa Global Index Medicus (Africa Index Medicus; search.bvsalud.org/ghl/?lang=en&submit=Search&where=REGIONAL; searched 21 May 2018).
IMEMR Global Index Medicus (Index Medicus for the Eastern Mediterranean Region; search.bvsalud.org/ghl/?lang=en&submit=Search&where=REGIONAL; searched 21 May 2018).
LILACS (Latin American and Caribbean Health Sciences Literature; lilacs.bvsalud.org/en; searched 21 May 2018).
PAHO/WHO Institutional Repository for Information Sharing (iris.paho.org/xmlui; searched 21 May 2018).
WHOLIS Global Index Medicus (WHO Library Database; search.bvsalud.org/ghl/?lang=en&submit=Search&where=REGIONAL; searched 21 May 2018).
WPRIM Global Index Medicus (Western Pacific Index Medicus; search.bvsalud.org/ghl/?lang=en&submit=Search&where=REGIONAL; searched 21 May 2018).
IMSEAR Global Index Medicus (Index Medicus for the South‐East Asian Region; search.bvsalud.org/ghl/?lang=en&submit=Search&where=REGIONAL; searched 21 May 2018).
IndMED (Indexing of Indian Medical Journals; indmed.nic.in/indmed.html; searched 21 May 2018).
Native Health Research Database (hscssl.unm.edu/nhd; searched 21 May 2018).
Searching other resources
We checked the reference lists of included studies and relevant reviews for further studies. We contacted authors of eligible studies and other related people for information about ongoing or unpublished studies we may have missed or, where necessary, to provide missing data (Dewey 2017a [pers comm]; Dewey 2017b [pers comm]; Moran 2016 [pers comm]; Stewart 2017 [pers comm]).
Data collection and analysis
Selection of studies
Two review authors (of ZH, AZB and RAS) independently assessed all records generated by the search strategy. First, they screened titles and abstracts of all records retrieved and short‐listed those deemed potentially relevant. Next, they obtained and assessed the full texts of all potentially relevant records, assessing each one against the inclusion criteria (see Criteria for considering studies for this review) before deciding on the final list of studies to be included. We resolved any disagreements through discussion or, if required, in consultation with a third review author (JKD).
We recorded our decisions in a PRISMA diagram (Moher 2009).
Data extraction and management
For eligible studies, two review authors (ZH, NGV, AZB and RAS) independently extracted data using a form designed for this review. We resolved any discrepancies through discussion with the entire group and documented these in the review. We completed a data collection form electronically and extracted and recorded the following information.
Study methods
Study design
Unit and method of allocation
Method of sequence generation
Masking of participants, personnel and outcome assessors
Participants
Location of study
Sample size
Age
Sex
Socioeconomic status (as defined by trialists and where such information was available)
Baseline prevalence of anaemia
Baseline BMI status
Inclusion and exclusion criteria
Intervention
Dose (SQ‐LNS providing less than 120 kcal/day; MQ‐LNS providing 120 to less than 250 kcal/day; and LQ‐LNS providing 250 to 500 kcal/day)
Formulation of LNS
Frequency of distribution of LNS to participants
Duration of intervention
Cointervention
Comparison group
No intervention or placebo
IFA
MMN supplements
MMP
Nutrition counselling
Outcomes
Primary and secondary outcomes, as outlined in the Types of outcome measures section
Exclusion of participants after randomisation and proportion of losses at follow‐up
We recorded both prespecified and non‐prespecified outcomes, although we did not use the latter to underpin the conclusions of the review.
We entered the data into Review Manager 5 (RevMan 5) software (Review Manager 2014).
Assessment of risk of bias in included studies
Using the criteria from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), and set out in Appendix 2, two review authors (NGV and JKD) independently assessed the risk of bias of each included study as high, low or unclear, across the following seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other potential sources of bias. We resolved any disagreement by discussion or by involving a third assessor (ZH). We also summarised the risk of bias within studies (across domains). To do this, we assessed the likely magnitude and direction of the bias in each of the aforementioned domains and considered whether they were likely to impact on the findings. We considered studies to be at high risk of bias if they had poor or unclear allocation concealment and either inadequate blinding or high/imbalanced losses to follow‐up. We explored the impact of the level of bias through a Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as risk ratios (RR) with 95% confidence intervals (CI).
Continuous data
For continuous data, we used the mean difference (MD) with 95% CI if outcomes were measured in the same way between studies. We used the standardised mean difference (SMD) with 95% CI to combine studies that measured the same outcome but used different measurement methods.
When some studies reported endpoint data and others reported change from baseline data (with errors), we combined these in the meta‐analysis, provided the outcomes were reported using the same scale.
Please refer to our protocol, Das 2017, and Table 3 for additional methods archived for use in future updates of this review.
1. Unused methods.
| Method | Approach |
| Measures of treatment effect |
Rates If rates represent events that could have occurred more than once per participant, we will report the rate difference using the methodologies described in Deeks 2011. |
| Unit of analysis issues | Cluster‐randomised studies Where possible, we will estimate the intra‐cluster correlation co‐efficient (ICC) from trials' original data sets and will report the design effect. We will use the methods set out in the Cochrane Handbook for Systematic Reviews of Interventions to calculate the adjusted sample sizes (Higgins 2011b). We will use an estimate of the ICC derived from the study (if possible), from a similar study or from a study of a similar population. If we use ICCs from other sources, we shall report this and conduct sensitivity analyses to investigate the effect of variation in the ICC (see Sensitivity analysis). If we identify both cluster‐RCTs and individually randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit (see Sensitivity analysis). |
| Assessment of reporting bias | If we include 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually, and use formal tests for funnel plot asymmetry. For continuous outcomes, we will use the test proposed by Egger 1997. For dichotomous outcomes, we will use the test proposed by Harbord 2006. If any of these tests detect asymmetry, or if it is suggested by a visual assessment, we will perform exploratory analyses to investigate it. |
| Subgroup analysis and investigation of heterogeneity | We will conduct the following subgroup analyses.
|
| Sensitivity analysis | We will conduct sensitivity analyses to assess the robustness of the results to the following.
|
ICC: intra‐class correlation coefficient.
Unit of analysis issues
Cluster‐randomised studies
Where possible, our plan was to estimate the intra‐class correlation coefficient (ICC) from the studies' original data sets and report the design effect, using the methods set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). However, we did not need to do this since we included only one cluster‐randomised study, Huybregts 2009 (C), and the trialists had already presented the analysis having appropriately adjusted for clustering. Consequently, we included this study in the same analyses as individually randomised studies. We considered it reasonable to combine the results because there was little heterogeneity between the study designs, and it was unlikely that the effect of intervention and the choice of randomisation unit would interact.
Studies with more than two treatment groups
For studies with more than two intervention groups (multi‐arm trials) and a single control group, we included the directly relevant arms only. When we identified studies with various relevant arms, we followed the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), and we combined the groups to make a single pair‐wise comparison (if possible). If the control group was shared by two or more intervention groups, we divided the control group (events and total population) over the number of relevant subgroup categories to avoid double counting the participants in the control group. We reported details related to multiple arms in the Characteristics of included studies tables.
Dealing with missing data
We attempted to obtain missing data from the study investigators. If this was not possible, we reported the data as missing and did not attempt to impute values. We describe the missing data, including dropouts, in the 'Risk of bias' tables, beneath the Characteristics of included studies tables. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis (i.e. we attempted to include all participants randomised to each group in the analyses and all participants analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention). The denominator for each outcome in each study was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed methodological heterogeneity by examining the methodological characteristics and 'Risk of bias' of the studies, and we assessed clinical heterogeneity by examining the similarity between the types of participants, interventions and outcomes.
For statistical heterogeneity, we examined the forest plots of meta‐analyses looking for heterogeneity among studies, and used the I² statistic, Tau² statistic and Chi² test to quantify the level of heterogeneity among the studies in each analysis. If we identified moderate or substantial heterogeneity, we explored it by prespecified subgroup analyses (see Subgroup analysis and investigation of heterogeneity). We regarded heterogeneity as substantial if the value of the I² statistic was greater than 50%, and either Tau² was greater than zero or there was a low P value (< 0.10) in the Chi² test for heterogeneity. Due to limited number of studies in each analysis, we could not conduct all the prespecified subgroup analysis, but for future updates in case of heterogeneity, we will perform the prespecified subgroup analysis (see Subgroup analysis and investigation of heterogeneity).
We advise caution in the interpretation of analyses with high degrees of heterogeneity.
Assessment of reporting biases
We did not find 10 studies reporting a single outcome and hence could not assess reporting bias. For methods to assess reporting bias in future updates of this review, please refer to our protocol, Das 2017, and Table 3.
Data synthesis
We carried out statistical analysis using RevMan 5 (Review Manager 2014). We combined the data using a random‐effects model, considering the differences in the intervention, using the Mantel‐Haenszel method for dichotomous outcomes and the inverse‐variance method for continuous outcomes. We only used a fixed‐effect model as a sensitivity analysis (if it was likely to be plausible); see Sensitivity analysis. We conducted a meta‐analysis where studies were examining the same intervention, and the studies' populations and methods were judged to be sufficiently similar.
We presented the results as the average treatment effect with 95% CI and the estimates of Tau², Chi² and I² (Deeks 2011).
Where it was not appropriate to conduct a meta‐analysis, we described the results as reported by the study authors.
Subgroup analysis and investigation of heterogeneity
Due to limitations of the data, we were only able to conduct the following subgroup analysis for LNS versus MMN: energy content. Please see Das 2017, and Table 3 for additional subgroup analyses archived for use in future updates of this review.
Sensitivity analysis
We were unable to conduct our preplanned sensitivity analyses due to the limited number of studies included in this review. We have archived these for use in future updates of this review (Das 2017; Table 3).
'Summary of findings' table
For the assessment across studies included in the review, two review authors (JKD and RAS) independently rated the quality of the evidence of each outcome as one of four levels (high, moderate, low or very low), using the GRADE approach (Balshem 2010), which involves consideration of within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias. A third review author (ZAB) helped to resolve any disagreements.
We present the GRADE quality ratings for our outcomes, along with estimates of relative effects, number of participants and studies contributing data for those outcomes, in 'Summary of findings' tables, which we prepared using GRADE profiler software (GRADEpro GDT 2015). We prepared separate tables for each comparison. Table 1 sets out the findings for the LNS versus IFA comparison while Table 2 sets out the findings for the LNS versus MMN comparison. We included the following outcomes in both tables: gestational weight gain per week, maternal anaemia at term or near term, maternal mortality, low birth weight, length at birth, SGA and preterm births.
Summary of findings for the main comparison. Summary of findings: lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA).
| Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA) | |||||
|
Patient or population: pregnant women Settings: community Intervention: LNS Comparison: IFA | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| IFA | LNS | ||||
| Gestational weight gain per week (from < 20 weeks gestation till the time of delivery) | 0 | The mean gestational weight gain per week in the intervention group was 0.46 standard deviations higher (0.44 lower to 1.36 higher) | — | 3539 participants (2 studies) |
⊕⊕⊕⊝ Moderatea |
| Maternal anaemia at term or near term (haemoglobin (Hb) less than 110 g/L) | 38/270 | 88/266 | RR 2.35 (1.67 to 3.30) | 536 participants (1 study) | ⊕⊕⊕⊝ Moderatea |
| Maternal mortality (measured at six weeks postpartum) | 6/3773 | 2/1855 | RR 0.53 (0.12 to 2.41) | 5628 participants (3 studies) |
⊕⊕⊕⊝ Moderatea |
| Low birth weight (< 2500 g) | 1100/3241 | 396/1585 | RR 0.87 (0.72 to 1.05) | 4826 participants (3 studies) | ⊕⊕⊕⊝ Moderatea |
| Length at birth (in cm) | The mean length at birth in the control groups ranged from 47.4 cm to 49.5 cm | The mean length at birth in the intervention groups was, on average, 0.24 cm longer (0.11 longer to 0.36 longer) | — | 4986 participants (3 studies) | ⊕⊕⊕⊝ Moderatea |
| Small‐for‐gestational age | 1943/3240 | 772/1583 | RR 0.94 (0.89 to 0.99) | 4823 participants (3 studies) | ⊕⊕⊕⊝ Moderatea |
| Preterm births (births before 37 weeks of gestation) | 426/3290 | 186/1634 | RR 0.94 (0.80 to 1.11) | 5924 participants (3 studies) | ⊕⊕⊕⊝ Moderatea |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval; IFA: Iron folic acid; LNS: Lipid‐based nutrient supplements; RR: Risk ratio; SMD: Standardised mean difference | |||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded by one level (from high to moderate) due to study limitations (lack of blinding of participants and personnel).
Summary of findings 2. Summary of findings: lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN).
| Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN) | |||||
|
Patient or population: pregnant women Settings: community Intervention: LNS Comparison: MMN | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| MMN | LNS | ||||
| Gestational weight gain per week (from < 20 weeks gestation till the time of delivery) | One study found no difference in gestational weight gain per week between the two groups | — | 682 (1 study) |
⊕⊕⊕⊝ Moderatea | |
| Maternal anaemia at term or near term (haemoglobin (Hb) less than 110 g/L) | 69/291 | 88/266 | RR 1.40 (1.07 to 1.82) | 557 participants (1 study) |
⊕⊕⊕⊝ Moderatea |
| Maternal mortality (measured at six weeks postpartum) | Not measured | ||||
| Low birth weight (< 2500 g) | 150/1194 | 140/1210 | RR 0.92 (0.74 to 1.14) | 2404 participants (3 studies) | ⊕⊕⊕⊝ Moderateb |
| Length at birth (in cm) | The mean length at birth in the control groups ranged from 47.6 cm to 49.7 cm | The mean length at birth in the intervention groups was, on average, 0.20 cm longer (0.02 shorter to 0.42 longer0 | — | 2567 participants (3 studies) | ⊕⊕⊕⊝ Moderateb |
| Small‐for‐gestational age | 385/1190 | 371/1203 | RR 0.95 (0.84 to 1.07) | 2393 participants (3 studies) | ⊕⊕⊕⊝ Moderateb |
| Preterm births (births before 37 weeks of gestation) | 139/1318 | 160/1312 | RR 1.15 (0.93 to 1.42) | 2393 participants (3 studies) | ⊕⊕⊕⊝ Moderateb |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval; LNS: Lipid‐based nutrient supplements; MMN: Multiple micronutrients; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded by one level (from high to moderate) due to study limitations (lack of blinding of participants and personnel in Adu‐Afarwuah 2015). bDowngraded by one level (from high to moderate) due to study limitations (high risk of attrition bias in Huybregts 2009 (C)).
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies.
Results of the search
The search strategy yielded 4122 unique records for possible inclusion. We excluded 4071 records on the basis of title and abstract and a further 12 reports following full‐text screening. We included four studies (from 38 reports) in the review and identified one ongoing study. Figure 1 depicts the flow chart for selecting the studies.
1.
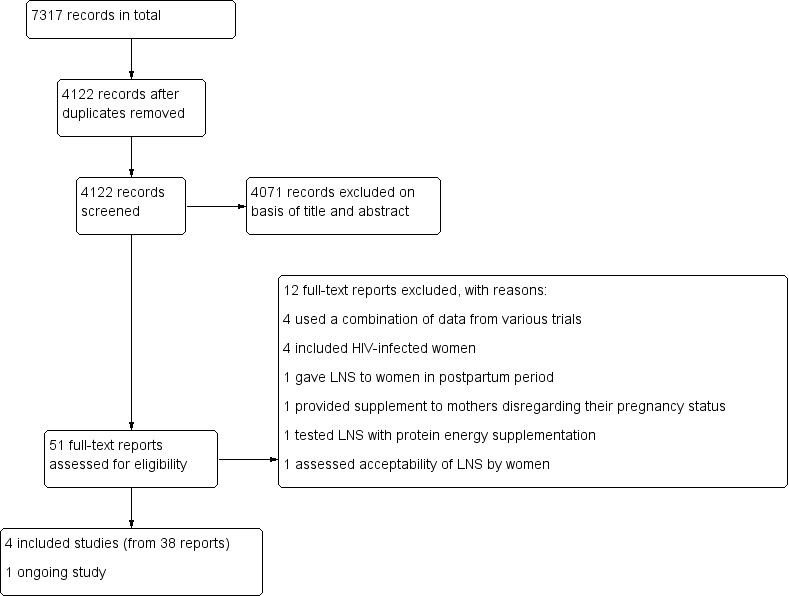
Study flow diagram.
Included studies
We included four studies in this review, all of which contributed data (Adu‐Afarwuah 2015; Ashorn 2015; Huybregts 2009 (C); Mridha 2016b).
Settings
The studies included in this review took place in four LMICs in Asia and Africa where maternal undernutrition is a public health problem: Ghana (Adu‐Afarwuah 2015), Malawi (Ashorn 2015), Burkina Faso (Huybregts 2009 (C)), and Bangladesh (Mridha 2016b). All studies were in stable community settings; we did not find any studies assessing LNS for pregnant women in emergency settings. The communities were semi‐urban in Ghana (Adu‐Afarwuah 2015), rural in Bangladesh and Burkina Faso (Huybregts 2009 (C); Mridha 2016b), and semi‐urban and partly rural communities in Malawi (Ashorn 2015).
Participants
Included studies involved a total of 8018 pregnant women at 20 weeks of gestation or less. Adu‐Afarwuah 2015 included only women aged 18 years or older, while Ashorn 2015 used a minimum cutoff age of 15. Two studies did not define a minimum age for enrolment (Huybregts 2009 (C); Mridha 2016b). The number of participants ranged from 1320 in Adu‐Afarwuah 2015 to 4011 in Mridha 2016.
Interventions
Of the four studies included in this review, two studies compared LNS to both IFA and MMN (Adu‐Afarwuah 2015; Ashorn 2015); one compared LNS to IFA (Mridha 2016b); and one compared LNS to MMN (Huybregts 2009 (C)). None of the included studies compared LNS with MNPs or nutrition education.
The interventions began during pregnancy and lasted up to six months postpartum. Follow‐up ranged from 36 weeks of gestation (Mridha 2016b) to 24 months postdelivery (Mridha 2016b). Community health visitors delivered the intervention in Huybregts 2009 (C) and Mridha 2016b, and the research team did so in Adu‐Afarwuah 2015 and Ashorn 2015.
In three studies, the energy content of LNS was 118 kcal/day (Adu‐Afarwuah 2015; Ashorn 2015; Mridha 2016b); one study, Huybregts 2009 (C), used LNS with 372 kcal/day. These also included macronutrients, micronutrients, vitamins (A, B, C, D, E and K), minerals and trace elements (such as iron, zinc, copper, etc.). The LNS could be mixed with home‐prepared food or eaten directly from the sachet. The composition of LNS used in each study is presented in the Characteristics of included studies table.
Outcomes
Primary outcomes
Of the maternal outcomes, studies reported data on maternal anthropometric status (gestational weight gain per week), maternal anaemia and adverse effects, while of the birth and infant outcomes, studies reported data on LBW, weight at birth, length at birth, SGA, preterm births and development outcomes.
Secondary outcomes
Of the maternal outcomes, included studies reported maternal adherence or compliance with LNS, while of the birth and infant outcomes, studies reported data on miscarriage and stillbirth, head circumference, MUAC, newborn stunting, underweight and neonatal death.
Our list of prespecified outcomes did not include maternal mortality or duration of gestation (Das 2017), but since three studies reported data on these outcomes, we decided to report the findings for this outcome in our review. See Differences between protocol and review.
Excluded studies
We excluded a total of 12 studies after full‐text screening: four studies that enrolled HIV‐infected women (Flax 2012; Flax 2014; Hampel 2018; Kayira 2012); one study that assessed the effect of postpartum provision of LNS on breast milk quality (Haber 2016); one study that assessed LNS plus protein energy supplementation (Johnson 2017); one study that was formative research assessing the acceptability of LNS (Young 2015); one study that reported food insecurity data (Adams 2017); one study that reported the willingness to pay (Adams 2018); one study that reported collective findings from two of the included trials on maternal plasma fatty acid status and lipid profile (Oaks 2017); one study that reported the factors associated with language and motor development collectively in four cohorts of children (Prado 2017); and one study that provided LNS to women regardless of pregnancy status (Schlossman 2017). For further detail, please see the Characteristics of excluded studies tables.
Ongoing studies
We identified one ongoing, cluster‐randomised, five‐arm, controlled trial (Fernald 2016). The sample size will comprise 25 communities in each of the five arms, with a total of 1250 pregnant women, 1250 children aged from birth to six months, and 1250 children aged from six to 18 months. The five intervention arms are as follows.
T0: existing programme with monthly growth monitoring and nutritional/hygiene education.
T1: T0 plus home visits for intensive nutrition counselling within a behaviour change framework.
T2: T1 plus LNS for children aged 6 months to 18 months old.
T3: T2 plus LNS supplementation of pregnant or lactating women.
T4: T1 plus intensive home‐visiting programme to support child development.
Primary outcomes will include child length/height‐for‐age z scores as well as mental, motor and social development outcomes. Secondary outcomes will include caregiver‐reported child morbidity, household food security and diet diversity, micronutrient status, and maternal knowledge of childcare, feeding practices and home stimulation practices.
See Characteristics of ongoing studies tables.
Risk of bias in included studies
See the 'Risk of bias' tables, beneath the Characteristics of included studies tables, for an assessment of the risk of bias for each included study, and Figure 2 and Figure 3 for an overall summary of the risk of bias of all included studies. We considered studies to be of high quality when we assessed them as being at low risk of bias for random sequence generation, low risk of bias for allocation concealment (selection bias) and low risk of bias for either blinding (performance or detection bias) or incomplete outcome data (attrition bias).
2.
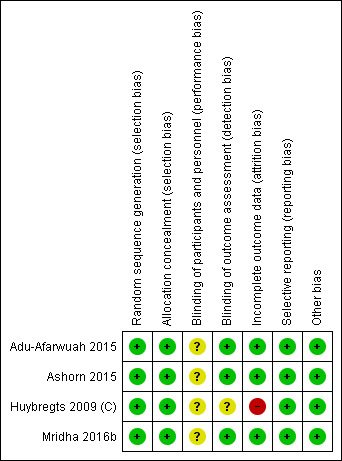
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
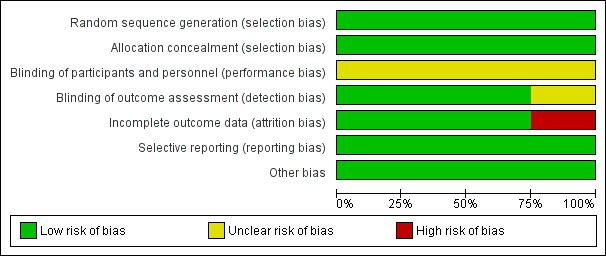
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
We assessed all four studies as having adequate methods for generating the randomisation sequence and rated them at low risk of bias (Adu‐Afarwuah 2015; Ashorn 2015; Huybregts 2009 (C); Mridha 2016b).
Allocation concealment
All four studies used computer‐generated numbers for randomisation and coded envelopes for allocation concealment, so we judged them to be at low risk of bias on this domain (Adu‐Afarwuah 2015; Ashorn 2015; Huybregts 2009 (C); Mridha 2016b).
Blinding
The studies did not blind participants as the consistency of the interventions was different (capsules for IFA or MMN versus sachets for LNS) (Adu‐Afarwuah 2015; Ashorn 2015; Mridha 2016b; Huybregts 2009 (C)). However, since the outcomes were objective but it was unclear whether the lack of blinding could have conferred any risk of bias, we rated all four studies as being at unclear risk of performance bias.
We rated three studies at low risk of detection bias (Adu‐Afarwuah 2015; Ashorn 2015; Mridha 2016b), and one study, Huybregts 2009 (C), at unclear risk of detection bias since it was not clear if the outcome assessment was blinded.
Incomplete outcome data
We considered studies with more than 20% loss to follow‐up of the total included participants to be inadequate in terms of completeness of outcome data. Three studies had less than 20% attrition, so we rated these at low risk of attrition bias (Adu‐Afarwuah 2015; Ashorn 2015; Mridha 2016b). One study had more than 20% attrition, so we judged it to be at high risk of attrition bias (Huybregts 2009 (C)).
Selective reporting
All included studies were registered and provided NCT numbers. We reviewed the protocols for each of the included studies and the methods section of the reports. We judged all four studies to be at low risk of reporting bias (Adu‐Afarwuah 2015; Ashorn 2015; Huybregts 2009 (C); Mridha 2016b). Given the small number of studies, we were not able to generate funnel plots to investigate the relationship between effect size and standard error (see Das 2017; Table 3).
Other potential sources of bias
As we identified no other potential sources of bias, we rated all four studies at low risk of bias on this domain (Adu‐Afarwuah 2015; Ashorn 2015; Huybregts 2009 (C); Mridha 2016b). The studies specified no role of funding agencies in implementation or analysis.
Effects of interventions
In this review, we have included four studies involving 8018 pregnant women. We have organised the results by the different comparisons and by primary and secondary outcomes. Most of the included studies focused on maternal anthropometric indices along with neonatal and infant anthropometric outcomes; a few reported on the other prespecified outcomes in the protocol (Das 2017). We could not conduct any sensitivity analysis due to the limited number of studies.
Comparison 1: LNS versus IFA
Maternal primary outcomes
Maternal anthropometric status: pooled study results
Two studies with 3539 participants reported data on this outcome (Adu‐Afarwuah 2015; Mridha 2016b). There was no significant difference between the LNS and IFA groups for maternal gestational weight gain per week (SMD 0.46, 95% CI −0.44 to 1.36; Tau2 = 0.42, Chi2 = 105.54, I2 = 99%; moderate‐quality evidence; Analysis 1.1). The high heterogeneity could be attributable to the differences in study settings: semi‐urban communities in Ghana (Adu‐Afarwuah 2015) and rural communities in Bangladesh (Mridha 2016b).
1.1. Analysis.
Comparison 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), Outcome 1 Gestational weight gain.
Maternal anaemia at term or near term: single study results
One study with 536 participants, Adu‐Afarwuah 2015, reported data showing a two‐fold increase in the prevalence of anaemia in the LNS group compared to the IFA group (RR 2.35, 95% CI 1.67 to 3.30; moderate‐quality evidence; Analysis 1.2).
1.2. Analysis.
Comparison 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), Outcome 2 Maternal anaemia.
Maternal mortality: pooled study results
Three studies with 5628 participants reported maternal mortality (Adu‐Afarwuah 2015; Ashorn 2015; Mridha 2016b). There was no significant difference in maternal mortality between the two groups (RR 0.53, 95% CI 0.12 to 2.41; I2 = 0%; moderate‐quality evidence; Analysis 1.3).
1.3. Analysis.
Comparison 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), Outcome 3 Maternal mortality.
Adverse effects: single study results
One study with 881 participants reported data on this outcome (Adu‐Afarwuah 2015). Adu‐Afarwuah 2015 defined adverse effects as one or more episodes of hospitalisation and did not find any significant difference in hospitalisation episodes between the LNS and IFA groups (59/440 hospitalisations in the LNS group compared to 44/441 hospitalisations in the IFA group, P = 0.11; Analysis 1.4).
1.4. Analysis.
Comparison 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), Outcome 4 Adverse effects.
Birth and infant primary outcomes
Low birth weight: pooled study results
Three studies with 4826 participants reported data on this outcome (Adu‐Afarwuah 2015; Ashorn 2015; Mridha 2016b). There was no significant difference in low birth weight between the two groups (RR 0.87, 95% CI 0.72 to 1.05; Tau2 = 0.01, Chi2 = 3.00, I2 = 33%; moderate‐quality evidence; Analysis 1.5; Figure 4).
1.5. Analysis.
Comparison 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), Outcome 5 Low birth weight (LBW).
4.
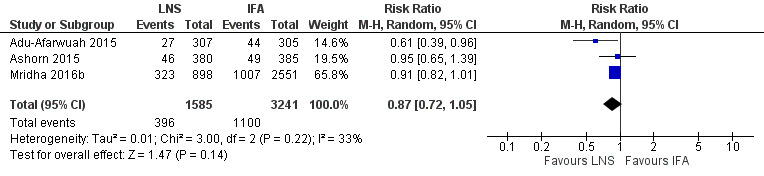
Forest plot of comparison: 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), outcome: 1.5 Low birth weight (LBW).
Weight at birth: pooled study results
Three studies with 5077 participants reported data on this outcome (Adu‐Afarwuah 2015; Ashorn 2015; Mridha 2016b). The birth weight (g) of neonates born to mothers consuming LNS was slightly higher than those consuming IFA (MD 53.28 g, 95% CI 28.22 to 78.33; Tau2 = 0.00, Chi2 = 1.18, I2 = 0%; moderate‐quality evidence; Analysis 1.6; Figure 5).
1.6. Analysis.
Comparison 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), Outcome 6 Weight at birth.
5.
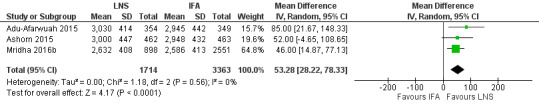
Forest plot of comparison: 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), outcome: 1.6 Weight at birth.
Length at birth: pooled study results
Three studies with 4986 participants reported data on this outcome (Adu‐Afarwuah 2015; Ashorn 2015; Mridha 2016b). The birth length (cm) of neonates born to mothers consuming LNS was slightly higher than those consuming IFA (MD 0.24 cm, 95% CI 0.11 to 0.36; Tau2 = 0.00, Chi2 = 1.47, I2 = 0%; moderate‐quality evidence; Analysis 1.7; Figure 6).
1.7. Analysis.
Comparison 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), Outcome 7 Length at birth.
6.
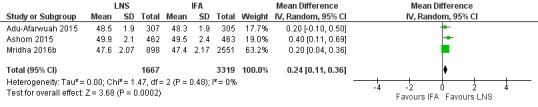
Forest plot of comparison: 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), outcome: 1.7 Length at birth.
SGA: pooled study results
Three studies with 4823 participants reported data on this outcome (Adu‐Afarwuah 2015; Ashorn 2015; Mridha 2016b). There was lower risk of SGA neonates being born to mothers who consumed LNS compared to those who consumed IFA (RR 0.94, 95% CI 0.89 to 0.99; Tau2 = 0.00, Chi2 = 0.98, I2 = 0%; moderate‐quality evidence; Analysis 1.8).
1.8. Analysis.
Comparison 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), Outcome 8 Small‐for‐gestational age (SGA).
Preterm births: pooled study results
Three studies with 4924 participants reported data on this outcome (Adu‐Afarwuah 2015; Ashorn 2015; Mridha 2016b). There was no significant difference in preterm births between the two groups (RR 0.94, 95% CI 0.80 to 1.11; Tau2 = 0.00, Chi2 = 0.73, I2 = 0%; moderate‐quality evidence; Analysis 1.9).
1.9. Analysis.
Comparison 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), Outcome 9 Preterm births.
Developmental outcomes: single study results
Three studies assessed developmental outcomes (Adu‐Afarwuah 2015; Ashorn 2015; Mridha 2016b).
Using maternal report at 12 months of children's age, Ashorn 2015 found that children in the LNS group achieved walking alone and waving goodbye earlier than children in the IFA group. Adu‐Afarwuah 2015 found that a greater percentage of children in the SQ‐LNS group were able to walk alone at 12 months of age compared to children in the IFA group. There was no impact on any of the gross motor development outcomes at 18 months of age in these two studies.
Mridha 2016b found that motor development and receptive language scores were higher for infants in the LNS group compared to the control group, at 18 months and 24 months of age. There was no difference in expressive language scores at 18 months of age; however, scores were better in the LNS group at 24 months of age compared to the control group. There was no difference in personal social scores and executive function score.
No other developmental outcomes were reported by any of the included studies, and there is very limited existing data on the effect of LNS on neurodevelopmental outcomes.
Maternal secondary outcomes
Duration of gestation: pooled study results
Three studies with 5033 participants reported duration of gestation (Adu‐Afarwuah 2015; Ashorn 2015; Mridha 2016b). There was a significant increase in the duration of gestation in the LNS group compared to the IFA group (MD 0.18 weeks, 95% CI 0.04 to 0.32; Tau2 = 0.00, Chi2 = 0.34, I2 = 0%; moderate‐quality evidence; Analysis 1.10).
1.10. Analysis.
Comparison 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), Outcome 10 Duration of gestation.
Maternal adherence or compliance with LNS
Three studies with 4826 participants reported maternal LNS acceptability and adherence (Adu‐Afarwuah 2015; Ashorn 2015; Mridha 2016b). LNS was acceptable to pregnant women in all three studies. However, qualitative findings from these studies suggest that acceptability and adherence may be interlinked, complex and context‐related, and sustained consumption may require tailoring interventions by context, with a focus on programmatic barriers and incorporating reminder techniques.
No studies reported data on the following maternal secondary outcomes for this comparison: maternal Hb at term or near term and maternal satisfaction with LNS.
Birth and infant secondary outcomes
Miscarriage and stillbirths: pooled study results
Two studies with 4714 participants reported data on miscarriages (Adu‐Afarwuah 2015; Mridha 2016b). There was no significant difference in miscarriages between the two groups (RR 0.87, 95% CI 0.66 to 1.14; Tau2 = 0.00, Chi2 = 0.35, I2 = 0%; Analysis 1.11).
1.11. Analysis.
Comparison 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), Outcome 11 Miscarriage.
Three studies with 5575 participants reported data on stillbirths (Adu‐Afarwuah 2015; Ashorn 2015; Mridha 2016b). There was no significant difference in stillbirths between the two groups (RR 1.14, 95% CI 0.52 to 2.48; Tau2 = 0.29, Chi2 = 5.38, I2 = 63%; Analysis 1.12).
1.12. Analysis.
Comparison 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), Outcome 12 Stillbirth.
Head circumference: pooled study results
Three studies with 4982 participants reported data on this outcome (Adu‐Afarwuah 2015; Ashorn 2015; Mridha 2016b). There was no significant difference in head circumference (cm) between the two groups (MD 0.20 cm, 95% CI 0.20 to 0.20; 2 studies, 4057 participants; Tau2 = 0.00, Chi2 = 0.000, I2 = 0%; Analysis 1.13). However, the head circumference z score was higher in the LNS group (MD 0.11, 95% CI 0.04 to 0.18; 3 studies, 4982 participants; Tau2 = 0.00, Chi2 = 2.20, I2 = 9%; Analysis 1.14).
1.13. Analysis.
Comparison 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), Outcome 13 Head circumference.
1.14. Analysis.
Comparison 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), Outcome 14 Head circumference z score.
MUAC: pooled study results
Two studies with 4374 participants reported data on this outcome (Ashorn 2015; Mridha 2016b). There was no significant difference in MUAC between the two groups (MD 0.12 cm, 95% CI −0.02 to 0.26; Tau2 = 0.01, Chi2 = 3.97, I2 = 75%; Analysis 1.15).
1.15. Analysis.
Comparison 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), Outcome 15 Mid‐upper‐arm circumference (MUAC).
Stunting at any time within the first six months: pooled study results
Two studies with 4166 participants reported data on newborn stunting (Ashorn 2015; Mridha 2016b). Newborn stunting was lower in the LNS group compared to the IFA group (RR 0.82, 95% CI 0.71 to 0.94; Tau2 = 0.00, Chi2 = 0.13, I2 = 0%; Analysis 1.16).
1.16. Analysis.
Comparison 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), Outcome 16 Newborn stunting.
Underweight at any time within the first six months: pooled study results
Two studies with 4174 participants reported data on newborn underweight (Ashorn 2015; Mridha 2016b). There was no significant difference in newborn underweight between the two groups (RR 0.84, 95% CI 0.63 to 1.13; Tau2 = 0.03, Chi2 = 1.70, I2 = 41%; Analysis 1.17).
1.17. Analysis.
Comparison 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), Outcome 17 Newborn underweight.
Neonatal death: pooled study results
Three studies with 7172 participants reported data on neonatal death (Adu‐Afarwuah 2015; Ashorn 2015; Mridha 2016b). There was no significant difference between the groups for neonatal death (RR 0.72, 95% CI 0.47 to 1.10; Tau2 = 0.00, Chi2 = 2.70, I2 = 0%), including early neonatal death (RR 0.70, 95% CI 0.45 to 1.09; Tau2 = 0.00, Chi2 = 1.86, I2 = 0%), and late neonatal death (RR 0.96, 95% CI 0.14 to 6.51; Tau2 = 0.00, Chi2 = 0.75, I2 = 0%). See Analysis 1.18.
1.18. Analysis.
Comparison 1 Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA), Outcome 18 Neonatal death.
No studies reported data on the following birth and infant secondary outcomes for this comparison: wasting at any time within the first six months and infant mortality.
Comparison 2: LNS versus MMN
Maternal primary outcomes
Maternal anthropometric status: single study results
One study with 682 participants reported data on gestational weight gain per week (Adu‐Afarwuah 2015), showing no significant difference in gestational weight gain per week between the two groups (weight gain of 0.2 kg/week in both groups; Analysis 2.1).
2.1. Analysis.
Comparison 2 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN), Outcome 1 Gestational weight gain per week.
Maternal anaemia at term or near term: single study results
One study with 557 participants reported data on anaemia (Adu‐Afarwuah 2015), showing increased anaemia in the LNS group when compared to the MMN group (RR 1.40, 95% CI 1.07 to 1.82; moderate‐quality evidence; Analysis 2.2).
2.2. Analysis.
Comparison 2 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN), Outcome 2 Maternal anaemia.
Adverse effects: single study results
One study with 879 participants reported data on this outcome (Adu‐Afarwuah 2015). Adu‐Afarwuah 2015 defined adverse effects as one or more episodes of hospitalisation and did not find any significant difference in hospitalisation episodes between the LNS and MMN groups (59/440 hospitalisations in the LNS group compared to 50/439 hospitalisations in the MMN group, P = 0.36; Analysis 2.3).
2.3. Analysis.
Comparison 2 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN), Outcome 3 Adverse events.
No studies reported data on maternal mortality.
Birth and infant primary outcomes
Low birth weight: pooled study results
Three studies with 2404 participants reported data on this outcome (Adu‐Afarwuah 2015; Ashorn 2015; Huybregts 2009 (C)). There was no significant difference in low birth weight between the two groups (RR 0.92, 95% CI 0.74 to 1.14; Tau2 = 0.00, Chi2 = 0.10, I2 = 0%; moderate‐quality evidence; Analysis 2.4).
2.4. Analysis.
Comparison 2 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN), Outcome 4 LBW.
Weight at birth: pooled study results
Three studies with 2573 participants reported data on this outcome (Adu‐Afarwuah 2015; Ashorn 2015; Huybregts 2009 (C)). The birth weight (g) of neonates born to mothers consuming LNS was no different than the birth weight of neonates born to mothers consuming MMN (MD 23.67 g, 95% CI −10.53 to 57.86; Tau2 = 0.00, Chi2 = 0.25, I2 = 0%; moderate‐quality evidence; Analysis 2.5).
2.5. Analysis.
Comparison 2 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN), Outcome 5 Weight at birth.
Length at birth: pooled study results
Three studies with 2567 participants reported data on this outcome (Adu‐Afarwuah 2015; Ashorn 2015; Huybregts 2009 (C)). The birth length (cm) of neonates born to mothers consuming LNS was no different than the birth length of neonates born to mothers consuming MMN (MD 0.20 cm, 95% CI −0.02 to 0.42; Tau2 = 0.01, Chi2 = 3.26, I2 = 39%; moderate‐quality evidence; Analysis 2.6).
2.6. Analysis.
Comparison 2 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN), Outcome 6 Length at birth.
SGA: pooled study results
Three studies with 2392 participants reported data on this outcome (Adu‐Afarwuah 2015; Ashorn 2015; Huybregts 2009 (C)). There was no significant difference in SGA neonates between the two groups (RR 0.95, 95% CI 0.84 to 1.07; Tau2 = 0.00, Chi2 = 0.85, I2 = 0%; moderate‐quality evidence; Analysis 2.7).
2.7. Analysis.
Comparison 2 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN), Outcome 7 SGA.
Preterm births: pooled study results
Three studies with 2630 participants reported data on this outcome (Adu‐Afarwuah 2015; Ashorn 2015; Huybregts 2009 (C)). There was no significant difference in preterm births between the two groups (RR 1.15, 95% CI 0.93 to 1.42; Tau2 = 0.00, Chi2 = 1.93, I2 = 0%; moderate‐quality evidence; Analysis 2.8).
2.8. Analysis.
Comparison 2 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN), Outcome 8 Preterm births.
No studies reported data on developmental outcomes.
Maternal secondary outcomes
Duration of gestation: pooled study results
Three studies with 2740 participants reported data on this outcome (Adu‐Afarwuah 2015; Ashorn 2015; Huybregts 2009 (C)). There was no significant difference in duration of gestation between the two groups (MD −0.07, 95% CI −0.26 to 0.12; Tau2 = 0.00, Chi2 = 0.98, I2 = 0%; Analysis 2.9)
2.9. Analysis.
Comparison 2 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN), Outcome 9 Duration of gestation.
No studies reported data on any of the maternal secondary outcomes for this comparison: maternal Hb at term or near term; maternal satisfaction with LNS; and maternal adherence or compliance with LNS.
Birth and infant secondary outcomes
Head circumference: pooled study results
Two studies with 1627 participants reported data on this outcome (Adu‐Afarwuah 2015; Huybregts 2009 (C)). There was no significant difference in head circumference (cm) between the two groups (MD 0.08 cm, 95% CI −0.16 to 0.31; Tau2 = 0.02, Chi2 = 2.50, I2 = 60%; Analysis 2.10).
2.10. Analysis.
Comparison 2 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN), Outcome 10 Head circumference.
Two studies with 1549 participants reported data on head circumference as measured by z scores (Adu‐Afarwuah 2015; Ashorn 2015). There was no significant difference in head circumference z scores between the two groups (MD 0.10, 95% CI −0.01 to 0.21; Tau2 = 0.00, Chi2 = 0.28, I2 = 0%; Analysis 2.11).
2.11. Analysis.
Comparison 2 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN), Outcome 11 Head circumference z score.
MUAC: pooled study results
Two studies with 1939 participants reported data on this outcome (Ashorn 2015; Huybregts 2009 (C)). The MUAC (cm) was not significantly different between the two groups (MD 0.07 cm, 95% CI −0.01 to 0.16; Tau2 = 0.00, Chi2 = 0.36, I2 = 0%; Analysis 2.12).
2.12. Analysis.
Comparison 2 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN), Outcome 12 MUAC.
Stunting at any time within the first six months: single study results
One study with 729 participants reported data on stunting (Ashorn 2015). There was no significant difference in newborn stunting between the two groups (RR 1.06, 95% CI 0.75 to 1.51; Analysis 2.13).
2.13. Analysis.
Comparison 2 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN), Outcome 13 Newborn stunting.
Underweight at any time within the first six months: single study results
One study with 737 participants reported data on this outcome (Ashorn 2015). There was no significant difference in newborn underweight between the two groups (RR 0.78, 95% CI 0.46 to 1.33; Analysis 2.14).
2.14. Analysis.
Comparison 2 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN), Outcome 14 Newborn underweight.
Neonatal death: single study results
One study with 1175 participants reported data on neonatal death (Huybregts 2009 (C)). There was no significant difference in neonatal mortality between the two groups (RR 0.88, 95% CI 0.36 to 2.15; Analysis 2.15).
2.15. Analysis.
Comparison 2 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN), Outcome 15 Neonatal death.
No studies reported data on the following birth and infant secondary outcomes for this comparison: miscarriage and stillbirths; wasting at any time within the first six months; and infant mortality.
Subgroup analysis by energy content
There was no significant difference in the subgroup analysis according to the energy content of LNS. See Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.6; Analysis 3.7; Analysis 3.8; Analysis 3.9.
3.1. Analysis.
Comparison 3 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN): subgrouped by energy content, Outcome 1 LBW.
3.2. Analysis.
Comparison 3 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN): subgrouped by energy content, Outcome 2 Weight at birth.
3.3. Analysis.
Comparison 3 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN): subgrouped by energy content, Outcome 3 Length at birth.
3.4. Analysis.
Comparison 3 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN): subgrouped by energy content, Outcome 4 SGA.
3.5. Analysis.
Comparison 3 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN): subgrouped by energy content, Outcome 5 Preterm births.
3.6. Analysis.
Comparison 3 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN): subgrouped by energy content, Outcome 6 Duration of gestation.
3.7. Analysis.
Comparison 3 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN): subgrouped by energy content, Outcome 7 Head circumference.
3.8. Analysis.
Comparison 3 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN): subgrouped by energy content, Outcome 8 MUAC.
3.9. Analysis.
Comparison 3 Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN): subgrouped by energy content, Outcome 9 Neonatal death: LQ‐LNS.
Discussion
Summary of main results
This review evaluated the effects of LNS in pregnant women and its impact on maternal, birth and infant outcomes. It includes four studies (8018 pregnant women); one study compares LNS versus IFA; one, LNS versus MMN; and two, LNS versus IFA or MMN. All studies evaluated SQ‐LNS with the except one study that evaluated the effect of LQ‐LNS (Huybregts 2009 (C)). We did not find any study comparing LNS with nutritional counselling.
Compared to IFA, LNS had a small, beneficial effect on birth weight, birth length, SGA and newborn stunting. However, there was no evidence of an effect for maternal gestational weight gain, low birth weight, miscarriage, stillbirth, preterm delivery, neonatal death and maternal mortality. One study reported adverse effects, showing no difference between the LNS and IFA groups. Findings for the developmental outcomes were scarce and not sufficient to draw any firm conclusions. Two studies reported on infant gross motor development, showing a small, positive impact on developmental outcomes at 12 months in the LNS group compared to the IFA group; there was no difference between the two groups at 18 months. One study showed a small, positive impact on language scores at 24 months, in favour of the LNS group. None of the included studies reported any of the other developmental outcomes.
Readers should interpret the beneficial findings of this review with caution due to the limited number of studies in the analyses and the fact that most of the evidence is driven by a single study, Mridha 2016b, conducted in a community‐based setting in Bangladesh, with a sample of 4011 pregnant women (mean age = 21.6 years).
When compared to MMN, there was no evidence of an effect of LNS on any of the reported maternal, birth or infant outcomes, and both interventions were found to be comparable. Only one study reported adverse effects and found no difference between the LNS and MMN groups.
Both IFA and MMN had a significant impact on maternal anaemia when compared to LNS.
The effect of LNS might differ depending on some potential, biologically plausible effect modifiers, including maternal baseline nutrition status, household food insecurity, household assets, maternal age and height, sex of the child, and time of year at birth (Mridha 2016b). We did not find any studies for LNS given to pregnant women in emergency settings.
Due to the limited number of included studies, we were unable to conduct our preplanned subgroup and sensitivity analyses (Das 2017), so we could not explore the most appropriate composition, frequency and duration of LNS.
Overall completeness and applicability of evidence
This review summarises findings from four studies (38 reports). All studies were published in the decade preceding publication of this review, with the oldest one dating to 2009. All studies took place in LMICs, and none of the included studies were in emergency settings. Of the four included studies, one compared LNS to IFA, one compared LNS to MMN, and two compared LNS to both IFA and MMN. We did not find any study comparing LNS with nutritional counselling. Three of the included studies provided SQ‐LNS, while one provided LQ‐LNS. The findings of this review may be generalisable to pregnant women in African and South Asian countries, since all of the studies are from community‐based settings in these regions. Findings are driven by one large (N = 4011), cluster‐randomised, community‐based trial from Bangladesh; however, the direction of effect for most outcomes is similar to other studies. We could not conduct subgroup analyses by baseline anaemia status, baseline BMI status, delivery strategy, duration of intervention or setting, due to the limited number of included studies.
Quality of the evidence
We considered the included studies to be of medium to high quality as regards allocation concealment and blinding of assessors. It was not possible to blind participants in all of the included studies due to the nature of the intervention (i.e. IFA and MMN were in tablet form while LNS was in the form of sachets). One study had more than 20% attrition, which may have affected the outcomes.
We judged all outcomes to be of moderate quality due to study limitations, high risk of attrition bias in Huybregts 2009 (C), and unclear risk of bias due to lack of blinding of participants and personnel.
Potential biases in the review process
We identified several potential biases in the review process, which we minimised in two ways: two review authors independently assessed the eligibility of studies for inclusion and extracted data, two review authors independently conducted the 'Risk of bias' assessments and entered the data into RevMan 5 (Review Manager 2014). However, subjective judgements are possible in these reviews, and others may reach different decisions regarding eligibility and risk of bias. We would encourage readers to examine the Characteristics of included studies tables to assist in the interpretation of results.
Agreements and disagreements with other studies or reviews
To our knowledge this is the only comprehensive review evaluating the effects of LNS supplementation during pregnancy. Findings from this review suggest a small, positive impact on birth and newborn outcomes (birth weight, birth length, SGA and newborn stunting). However, the beneficial findings of this review should be interpreted with caution since the evidence is from a small number of studies, with one large‐scale study in community settings in Bangladesh driving most of the impact, and effect sizes are too small to propose any concrete recommendation for practice.
Authors' conclusions
Implications for practice.
Findings from this review suggest that compared to IFA, LNS supplementation during pregnancy improves birth weight and birth length and reduces SGA and newborn stunting, but it does not impact on LBW, preterm birth, miscarriage, head circumference, underweight, neonatal death or infant mortality. These findings are consistent with evidence from MMN supplementation alone, which, when compared to IFA, reduces SGA and LBW (Haider 2015). All outcomes were between LNS and MMN, suggesting no additional benefits of LNS. Overall, findings suggest limited effect of LNS compared to IFA, but this effect diminishes when compared to MMN supplementation. Findings from this review should be interpreted with caution since the evidence comes from only a few studies, with one‐large scale study (conducted in community settings in Bangladesh) driving most of the impact. Furthermore, effect sizes are too small to propose any concrete recommendations for practice.
Existing evidence suggest that the magnitude of the effect of LNS is small and might differ depending on some potential, biologically plausible, effect modifiers, including household food insecurity, household assets, maternal age and height, sex of the child, and time of year at birth (Mridha 2016b). Futhermore, the findings from Adu‐Afarwuah 2015 show that compared to IFA and MMN, LNS in primiparous women improves birth length, birth weight, LBW and SGA; there was no evidence of an effect in multiparous women. These findings need further consideration and cautious evaluation. LNS may have a role in emergency settings, but this needs to be explored in future trials.
Implications for research.
The results of our review provide a number of implications for future research. First, there are no existing data on the impact of LNS supplementation in emergency settings. Second, existing data on the impact of LNS on the neurodevelopmental outcomes are insufficient to draw firm conclusions. Future studies should standardise these outcome measures to enable pooling in meta‐analysis. In addition, future research should use a double‐blind approach along with a placebo product for the control group (where appropriate), and for interventions where blinding is not feasible, mask outcome assessors. There is a need to evaluate the preventive impact of LNS for longer durations of intervention and also late follow‐ups to capture the long‐term impact of nutrition interventions. Furthermore, the effect of LNS in vulnerable groups, including low socioeconomic groups, undernourished women, and other food insecure populations need to be explored further to identify potential effect modifications and interactions among these variables across population groups, and large‐scale studies would be required to address these in different countries and contexts.
There are no existing studies comparing LNS with nutritional counselling. Future research should focus on evaluating the relative effectiveness and cost‐effectiveness of using commercially available products versus nutrition education to promote homemade, energy‐dense food to prevent malnutrition.
Acknowledgements
We are grateful to the Cochrane Developmental, Psychosocial and Learning Problems editorial team for their support in the preparation of this review. As part of the prepublication editorial process, four peers (a content editor, a statistical editor, and two referees external to the editorial team) commented on the review.
The review was partially developed during the WHO/Cochrane Collaboration/Cornell University Summer Institute for Systematic Reviews in Nutrition Global Policy Making, hosted at the Division of Nutritional Sciences, Cornell University, Ithaca, USA, from 25 July to 5 August 2016.
We acknowledge Kathryn Dewey (KD) who reviewed the draft meticulously and provided critical inputs especially on the technical aspects of the intervention and methodological aspects of the studies; she has been involved in these studies (KD is an author in three included studies). KD helped in finalising the review but did not have any role in the finalising the review's findings and conclusions.
We would like to acknowledge Yousaf Hadi for assistance with preliminary extractions and analysis.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials, in the Cochrane Library
#1[mh Lipids] #2fatty next acid* #3(Docosahexaenoic or Eicosapentaenoic) next acid* #4PUFA or PUFAs #5lipid* #6(omega next (3* or 6*)) #7(soy* or peanut or groundnut or whey or sesame or cashew or chickpea or oil*) #8{or #1‐#7} #9[mh "Dietary Supplements"] #10[mh "Food, fortified"] #11((diet* or food*) near/3 (fortif* or enrich* or supplement*)) #12(complement* near/3 (food* or feed*)) #13"Ready to use" #14"point of use" #15(RUSF or RUTF) #16(home* near/2 fortif*) #17{or #9‐#16} #18#8 and #17 #19lipid next based #20lipid* near/3 supplement* #21(lipid* near/3 nutrient*) #22(lipid* near/3 fortif*) #23(lipid* near/3 formulation*) #24(lipid* near/3 enrich*) #25(lipid* near/3 emuls*) #26(lipid* near/3 powder*) #27(lipid* near/3 spread*) #28(lipid* near/3 paste*) #29(Nutributter* or Plumpy*) #30(LNS or iLiNS) #31{or #19‐#30} #32#18 or #31 #33[mh Pregnancy] #34[mh "pregnant women"] #35[mh "Prenatal care"] #36[mh "Perinatal care"] #37(perinatal* or peri‐natal* or prenatal* or pre‐natal* or antenatal* or ante‐natal*) #38pregnan* #39trimester* #40[mh Mothers] #41(mother* or maternal*) #42{or #33‐#41} #43#32 and #42 in Trials
Ovid MEDLINE
1 exp Lipids/ 2 fatty acid$.tw,kf. 3 Docosahexaenoic acid$.tw,kf. 4 Eicosapentaenoic Acid$.tw,kf. 5 PUFA$.tw,kf. 6 lipid$.tw,kf. 7 (omega 3$ or omega 6$).tw,kf. 8 (soy$ or peanut or groundnut or whey or sesame or cashew or chickpea or oil$).tw,kf. 9 or/1‐8 10 Dietary Supplements/ 11 Food, fortified/ 12 ((diet$ or food$) adj3 (fortif$ or enrich$ or supplement$)).tw,kf. 13 (complement$ adj3 (food$ or feed$)).tw,kf. 14 "Ready to use".tw,kf. 15 (RUSF or RUTF).tw,kf. 16 "point of use".tw,kf. 17 (home$ adj2 fortif$).tw,kf. 18 or/10‐17 19 9 and 18 20 lipid based.tw,kf. 21 (lipid$ adj3 supplement$).tw,kf. 22 (lipid$ adj3 nutrient$).tw,kf. 23 (lipid$ adj3 fortif$).tw,kf. 24 (lipid$ adj2 formulation$).tw,kf. 25 (lipid$ adj3 enrich$).tw,kf. 26 (lipid$ adj2 emuls$).tw,kf. 27 (lipid$ adj3 powder$).tw,kf. 28 (lipid adj3 spread$).tw,kf. 29 (lipid$ adj3 paste$).tw,kf. 30 (Nutributter$ or Plumpy$).tw,kf. 31 (LNS$1 or iLiNS).tw,kf. 32 or/20‐31 33 19 or 32 34 Pregnancy/ 35 Pregnant Women/ 36 Prenatal care/ 37 Perinatal care/ 38 (perinatal$ or peri‐natal$ or prenatal$ or pre‐natal$ or antenatal$ or ante‐natal$).tw,kf. 39 pregnan$.tw,kf. 40 trimester$.tw,kf. 41 Mothers/ 42 (mother$ or maternal$).tw,kf. 43 or/34‐42 44 randomised controlled trial.pt. 45 controlled clinical trial.pt. 46 randomi#ed.ab. 47 placebo$.ab. 48 drug therapy.fs. 49 randomly.ab. 50 trial.ab. 51 groups.ab. 52 or/44‐51 53 exp animals/ not humans.sh. 54 52 not 53 55 33 and 43 and 54
Ovid MEDLINE In‐Process & Other Non‐Indexed Citations
1 fatty acid$.tw,kf. 2 Docosahexaenoic acid$.tw,kf. 3 Eicosapentaenoic Acid$.tw,kf. 4 PUFA$.tw,kf. 5 lipid$.tw,kf. 6 (omega 3$ or omega 6$).tw,kf. 7 (soy$ or peanut or groundnut or whey or sesame or cashew or chickpea or oil$).tw,kf. 8 or/1‐7 9 ((diet$ or food$) adj3 (fortif$ or enrich$ or supplement$)).tw,kf. 10 (complement$ adj3 (food$ or feed$)).tw,kf. 11 "Ready to use".tw,kf. 12 (RUSF or RUTF).tw,kf. 13 "point of use".tw,kf. 14 (home$ adj2 fortif$).tw,kf. 15 or/9‐14 16 lipid based.tw,kf. 17 (lipid$ adj3 supplement$).tw,kf. 18 (lipid$ adj3 nutrient$).tw,kf. 19 (lipid$ adj3 fortif$).tw,kf. 20 (lipid$ adj2 formulation$).tw,kf. 21 (lipid$ adj3 enrich$).tw,kf. 22 (lipid$ adj2 emuls$).tw,kf. 23 (lipid$ adj3 powder$).tw,kf. 24 (lipid adj3 spread$).tw,kf. 25 (lipid$ adj3 paste$).tw,kf. 26 (Nutributter$ or Plumpy$).tw,kf. 27 (LNS$1 or iLiNS).tw,kf. 28 or/16‐27 29 8 or (15 and 28) 30 (perinatal$ or peri‐natal$ or prenatal$ or pre‐natal$ or antenatal$ or ante‐natal$).tw,kf. 31 pregnan$.tw,kf. 32 trimester$.tw,kf. 33 (mother$ or maternal$).tw,kf. 34 or/30‐33 35 29 and 34 36 (random$ or control$ or group$ or cluster$ or placebo$ or trial$ or assign$ or prospectiv$ or meta‐analysis or systematic review or longitudinal$).tw,kf. 37 35 and 36
Ovid MEDLINE Epub Ahead of Print
1 fatty acid$.tw,kf. 2 Docosahexaenoic acid$.tw,kf. 3 Eicosapentaenoic Acid$.tw,kf. 4 PUFA$.tw,kf. 5 lipid$.tw,kf. 6 (omega 3$ or omega 6$).tw,kf. 7 (soy$ or peanut or groundnut or whey or sesame or cashew or chickpea or oil$).tw,kf. 8 or/1‐7 9 ((diet$ or food$) adj3 (fortif$ or enrich$ or supplement$)).tw,kf. 10 (complement$ adj3 (food$ or feed$)).tw,kf. 11 "Ready to use".tw,kf. 12 (RUSF or RUTF).tw,kf. 13 "point of use".tw,kf. 14 (home$ adj2 fortif$).tw,kf. 15 or/9‐14 16 lipid based.tw,kf. 17 (lipid$ adj3 supplement$).tw,kf. 18 (lipid$ adj3 nutrient$).tw,kf. 19 (lipid$ adj3 fortif$).tw,kf. 20 (lipid$ adj2 formulation$).tw,kf. 21 (lipid$ adj3 enrich$).tw,kf. 22 (lipid$ adj2 emuls$).tw,kf. 23 (lipid$ adj3 powder$).tw,kf. 24 (lipid adj3 spread$).tw,kf. 25 (lipid$ adj3 paste$).tw,kf. 26 (Nutributter$ or Plumpy$).tw,kf. 27 (LNS$1 or iLiNS).tw,kf. 28 or/16‐27 29 8 or (15 and 28) 30 (perinatal$ or peri‐natal$ or prenatal$ or pre‐natal$ or antenatal$ or ante‐natal$).tw,kf. 31 pregnan$.tw,kf. 32 trimester$.tw,kf. 33 (mother$ or maternal$).tw,kf. 34 or/30‐33 35 29 and 34 36 (random$ or control$ or group$ or cluster$ or placebo$ or trial$ or assign$ or prospectiv$ or meta‐analysis or systematic review or longitudinal$).tw,kf. 37 35 and 36
Embase Ovid
1 exp lipid/ 2 fatty acid$.tw,kw. 3 Docosahexaenoic acid$.tw,kw. 4 Eicosapentaenoic Acid$.tw,kw. 5 PUFA$.tw,kw. 6 lipid$.tw,kw. 7 (omega 3$ or omega 6$).tw,kw. 8 (soy$ or peanut or groundnut or whey or sesame or cashew or chickpea or oil$).tw,kw. 9 or/1‐8 10 dietary supplement/ 11 fortified food/ 12 ((diet$ or food$) adj3 (fortif$ or enrich$ or supplement$)).tw,kw. 13 (complement$ adj3 (food$ or feed$)).tw,kw. 14 "Ready to use".tw,kw. 15 (RUSF or RUTF).tw,kw. 16 "point of use".tw,kw. 17 (home$ adj2 fortif$).tw,kw. 18 or/10‐17 19 9 and 18 20 lipid based.tw,kw. 21 (lipid$ adj3 supplement$).tw,kw. 22 (lipid$ adj3 nutrient$).tw,kw. 23 (lipid$ adj3 fortif$).tw,kw. 24 (lipid$ adj2 formulation$).tw,kw. 25 (lipid$ adj3 enrich$).tw,kw. 26 (lipid$ adj2 emuls$).tw,kw. 27 (lipid$ adj3 powder$).tw,kw. 28 (lipid adj3 spread$).tw,kw. 29 (lipid$ adj3 paste$).tw,kw. 30 (Nutributter$ or Plumpy$).tw,kw. 31 (LNS$1 or iLiNS).tw,kw. 32 or/20‐31 33 19 or 32 34 exp pregnancy/ 35 exp prenatal care/ 36 exp perinatal care/ 37 (perinatal$ or peri‐natal$ or prenatal$ or pre‐natal$ or antenatal$ or ante‐natal$).tw,kw. 38 pregnan$.tw,kw. 39 trimester$.tw,kw. 40 exp mother/ 41 (mother$ or maternal$).tw,kw. 42 or/34‐41 43 33 and 42 44 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 45 human/ or normal human/ or human cell/ 46 44 and 45 47 44 not 46 48 43 not 47 49 Randomized controlled trial/ 50 controlled clinical trial/ 51 Single blind procedure/ 52 Double blind procedure/ 53 triple blind procedure/ 54 Crossover procedure/ 55 (crossover or cross‐over).tw. 56 ((singl$ or doubl$ or tripl$ or trebl$) adj1 (blind$ or mask$)).tw. 57 Placebo/ 58 placebo.tw. 59 prospective.tw. 60 factorial$.tw. 61 random$.tw. 62 assign$.ab. 63 allocat$.tw. 64 volunteer$.ab. 65 or/49‐64 66 48 and 65
CINAHL PLus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
S1 (MH "Lipids+") S2 TI (lipid*) or AB (lipid*) S3 TI(Docosahexaenoic acid*) OR AB(Docosahexaenoic acid*) S4 TI( Eicosapentaenoic acid*) OR AB( Eicosapentaenoic acid*) S5 TI(PUFA*) OR AB(PUFA* ) S6 TI(omega 3* or omega 6*) OR AB(omega 3* or omega 6* ) S7 TI (soy* or peanut or groundnut or whey or sesame or cashew or chickpea or oil*) or AB(soy* or peanut or groundnut or whey or sesame or cashew or chickpea or oil*) S8 TI(fatty acid*) OR AB(fatty acid* ) S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 S10 (MH "Dietary Supplements") S11 (MH "Dietary Supplementation") S12 (MH "Food, Fortified") S13 TI ((diet* or food*) n3 (fortif* or enrich* or supplement*)) OR AB((diet* or food*) n3 (fortif* or enrich* or supplement*)) S14 TI (complement* N3 (food* or feed*)) or AB (complement* N3 (food* or feed*)) S15 "Ready to use" S16 (RUSF or RUTF) S17 "point of use" S18 TI (home* N2 fortif*) OR AB(home* N2 fortif*) S19 S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 S20 S9 AND S19 S21 TI (lipid based) or AB (lipid based) S22 TI(lipid* N3 supplement*) OR AB( lipid* N3 supplement*) S23 TI(lipid* N3 nutrient*) OR AB(lipid* N3 nutrient* ) S24 TI(lipid* N3 fortif*) OR AB(lipid* N3 fortif*) S25 TI(lipid* N3 formulation*) OR AB(lipid* N3 formulation*) S26 TI(lipid* N3 enrich*) OR AB(lipid* N3 enrich*) S27 TI(lipid* N3 emuls*) OR AB(lipid* N3 emuls*) S28 TI(lipid* N3 powder*) OR AB(lipid* N3 powder*) S29 TI(lipid N3 spread*) OR AB(lipid N3 spread*) S30 TI(lipid* N3 paste*) OR AB(lipid* N3 paste*) S31 TI(Nutributter* or Plumpy*) OR AB(Nutributter* or Plumpy*) S32 Nutributter* or Plumpy* S33 TI(LNS or iLiNS) OR AB( LNS or iLiNS) S34 S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 S35 S20 OR S34 S36 (MH "Pregnancy+") S37 (MH "Pregnancy Trimesters+") S38 (MH "Prenatal Care") S39 (MH "Perinatal Care") S40 TI (perinatal* or peri‐natal* or prenatal* or pre‐natal* or antenatal* or ante‐natal*) or AB (perinatal* or peri‐natal* or prenatal* or pre‐natal* or antenatal* or ante‐natal*) S41 pregnan* S42 trimester* S43 (MH "Mothers+") S44 TI (mother* or maternal*) OR AB(mother* or maternal*) S45 S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 S46 S35 AND S45 S47 (MH "Clinical Trials+") S48 MH random assignment S49 (MH "Meta Analysis") S50 (MH "Crossover Design") S51 (MH "Quantitative Studies") S52 PT randomised controlled trial S53 PT Clinical trial S54 (trial* or control* or placebo*) S55 ("follow‐up study" or "follow‐up research") S56 (prospectiv* study or prospectiv* research) S57 (evaluat* N2 study or evaluat* N2 research) S58 (MH "Program Evaluation") S59 (MH "Treatment Outcomes") S60 TI(single N2 mask* or single N2 blind*) OR AB(single N2 mask* or single N2 blind*) S61 TI((doubl* N2 mask*) or (doubl* N2 blind*)) OR AB((doubl* N2 mask*) or (doubl* N2 blind*)) S62 TI ((tripl* N2 mask*) or (tripl* N2 blind*)) or ((trebl* N2 mask*) or (trebl* N2 blind*)) OR AB((tripl* N2 mask*) or (tripl* N2 blind*)) or ((trebl* N2 mask*) or (trebl* N2 blind*) S63 random* S64 S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 S65 S46 AND S64
Science Citation Index Web of Science (SCI)
# 19 #17 NOT #18 Indexes=SCI‐EXPANDED Timespan=All years # 18 TS= (RATS OR MICE OR SHEEP OR PIGS OR COWS OR CHICKS OR CHICKENS OR DUCK* ) Indexes=SCI‐EXPANDED Timespan=All years # 17 #16 AND #15 Indexes=SCI‐EXPANDED Timespan=All years # 16 TS= (RANDOM* OR TRIAL* OR CONTROL* OR PLACEBO* OR PROSPECTIV* OR LONGITUDINAL OR BLIND* OR GROUP* OR CLUSTER* OR meta‐analysis OR systematic review) Indexes=SCI‐EXPANDED Timespan=All years # 15 #14 AND #11 Indexes=SCI‐EXPANDED Timespan=All years # 14 #13 OR #12 Indexes=SCI‐EXPANDED Timespan=All years # 13 TS=(mother* or maternal*) Indexes=SCI‐EXPANDED Timespan=All years # 12 TS= (perinatal* or peri‐natal* or prenatal* or pre‐natal* or antenatal* or ante‐natal* or pregnan* or trimester*) Indexes=SCI‐EXPANDED Timespan=All years # 11 #6 or #10 Indexes=SCI‐EXPANDED Timespan=All years # 10 #9 OR #8 OR #7 Indexes=SCI‐EXPANDED Timespan=All years # 9 TS=(Nutributter* or Plumpy* OR LNS or iLiNS) Indexes=SCI‐EXPANDED Timespan=All years # 8 TS=(lipid* near/3 (supplement* or nutrient* or fortif* or formulation* or enrich* or emuls* or powder* or spread* or paste* )) Indexes=SCI‐EXPANDED Timespan=All years # 7 TS= ("lipid based") Indexes=SCI‐EXPANDED Timespan=All years # 6 #1 and #5 Indexes=SCI‐EXPANDED Timespan=All years # 5 #4 OR #3 OR #2 Indexes=SCI‐EXPANDED Timespan=All years # 4 TS=("Ready to use" or "point of use" or RUSF or RUTF or (home* near/2 fortif*)) Indexes=SCI‐EXPANDED Timespan=All years # 3 TS=(complement* near/3 (food* or feed*)) Indexes=SCI‐EXPANDED Timespan=All years # 2 TS= ((diet* or food*) near/3 (fortif* or enrich* or supplement*)) Indexes=SCI‐EXPANDED Timespan=All years # 1 TS=(lipid* OR "fatty acid*" OR ((Docosahexaenoic or Eicosapentaenoic) next acid*) or PUFA OR PUFAs OR "omega 3*" OR "omega 6*" OR soy* OR peanut* OR groundnut* OR whey* OR sesame* OR cashew* OR chickpea* OR oil* ) Indexes=SCI‐EXPANDED Timespan=All years
Social Sciences Citation Index Web of Science (SSCI)
#19 #17 NOT #18 Indexes=SSCI Timespan=All years # 18 TS= (RATS OR MICE OR SHEEP OR PIGS OR COWS OR CHICKS OR CHICKENS OR DUCK* ) Indexes=SSCI Timespan=All years # 17 #16 AND #15 Indexes=SSCI Timespan=All years # 16 TS= (RANDOM* OR TRIAL* OR CONTROL* OR PLACEBO* OR PROSPECTIV* OR LONGITUDINAL OR BLIND* OR GROUP* OR CLUSTER* OR meta‐analysis OR systematic review) Indexes=SSCI Timespan=All years # 15 #14 AND #11 Indexes=SSCI Timespan=All years # 14 #13 OR #12 Indexes=SSCI Timespan=All years # 13 TS=(mother* or maternal*) Indexes=SSCI Timespan=All years # 12 TS= (perinatal* or peri‐natal* or prenatal* or pre‐natal* or antenatal* or ante‐natal* or pregnan* or trimester*) Indexes=SSCI Timespan=All years # 11 #6 or #10 Indexes=SSCI Timespan=All years # 10 #9 OR #8 OR #7 Indexes=SSCI Timespan=All years # 9 TS=(Nutributter* or Plumpy* OR LNS or iLiNS) Indexes=SSCI Timespan=All years # 8 TS=(lipid* near/3 (supplement* or nutrient* or fortif* or formulation* or enrich* or emuls* or powder* or spread* or paste* )) Indexes=SSCI Timespan=All years # 7 TS= ("lipid based") Indexes=SSCI Timespan=All years # 6 #1 and #5 Indexes=SSCI Timespan=All years # 5 #4 OR #3 OR #2 Indexes=SSCI Timespan=All years # 4 TS=("Ready to use" or "point of use" or RUSF or RUTF or (home* near/2 fortif*)) Indexes=SSCI Timespan=All years # 3 TS=(complement* near/3 (food* or feed*)) Indexes=SSCI Timespan=All years # 2 TS= ((diet* or food*) near/3 (fortif* or enrich* or supplement*)) Indexes=SSCI Timespan=All years # 1 TS=(lipid* OR "fatty acid*" OR ((Docosahexaenoic or Eicosapentaenoic) next acid*) or PUFA OR PUFAs OR "omega 3*" OR "omega 6*" OR soy* OR peanut* OR groundnut* OR whey* OR sesame* OR cashew* OR chickpea* OR oil* ) Indexes=SSCI Timespan=All years
Conference Proceedings Citation Index ‐ Science Web of Science (CPCI‐S)
# 19 #17 NOT #18 Indexes=CPCI‐S Timespan=All years # 18 TS= (RATS OR MICE OR SHEEP OR PIGS OR COWS OR CHICKS OR CHICKENS OR DUCK* ) Indexes=CPCI‐S Timespan=All years # 17 #16 AND #15 Indexes=CPCI‐S Timespan=All years # 16 TS= (RANDOM* OR TRIAL* OR CONTROL* OR PLACEBO* OR PROSPECTIV* OR LONGITUDINAL OR BLIND* OR GROUP* OR CLUSTER* OR meta‐analysis OR systematic review) Indexes=CPCI‐S Timespan=All years # 15 #14 AND #11 Indexes=CPCI‐S Timespan=All years # 14 #13 OR #12 Indexes=CPCI‐S Timespan=All years # 13 TS=(mother* or maternal*) Indexes=CPCI‐S Timespan=All years # 12 TS= (perinatal* or peri‐natal* or prenatal* or pre‐natal* or antenatal* or ante‐natal* or pregnan* or trimester*) Indexes=CPCI‐S Timespan=All years # 11 #6 or #10 Indexes=CPCI‐S Timespan=All years # 10 #9 OR #8 OR #7 Indexes=CPCI‐S Timespan=All years # 9 TS=(Nutributter* or Plumpy* OR LNS or iLiNS) Indexes=CPCI‐S Timespan=All years # 8 TS=(lipid* near/3 (supplement* or nutrient* or fortif* or formulation* or enrich* or emuls* or powder* or spread* or paste* )) Indexes=CPCI‐S Timespan=All years # 7 TS= ("lipid based") Indexes=CPCI‐S Timespan=All years # 6 #1 and #5 Indexes=CPCI‐S Timespan=All years # 5 #4 OR #3 OR #2 Indexes=CPCI‐S Timespan=All years # 4 TS=("Ready to use" or "point of use" or RUSF or RUTF or (home* near/2 fortif*)) Indexes=CPCI‐S Timespan=All years # 3 TS=(complement* near/3 (food* or feed*)) Indexes=CPCI‐S Timespan=All years # 2 TS= ((diet* or food*) near/3 (fortif* or enrich* or supplement*)) Indexes=CPCI‐S Timespan=All years # 1 TS=(lipid* OR "fatty acid*" OR ((Docosahexaenoic or Eicosapentaenoic) next acid*) or PUFA OR PUFAs OR "omega 3*" OR "omega 6*" OR soy* OR peanut* OR groundnut* OR whey* OR sesame* OR cashew* OR chickpea* OR oil* ) Indexes=CPCI‐S Timespan=All years
Conference Proceedings Citation Index Social Science & Humanities Web of Science (CPCI‐SS&H)
# 19 #17 NOT #18 Indexes=CPCI‐SSH Timespan=All years # 18 TS= (RATS OR MICE OR SHEEP OR PIGS OR COWS OR CHICKS OR CHICKENS OR DUCK* ) Indexes=CPCI‐SSH Timespan=All years # 17 #16 AND #15 Indexes=CPCI‐SSH Timespan=All years # 16 TS= (RANDOM* OR TRIAL* OR CONTROL* OR PLACEBO* OR PROSPECTIV* OR LONGITUDINAL OR BLIND* OR GROUP* OR CLUSTER* OR meta‐analysis OR systematic review) Indexes=CPCI‐SSH Timespan=All years # 15 #14 AND #11 Indexes=CPCI‐SSH Timespan=All years # 14 #13 OR #12 Indexes=CPCI‐SSH Timespan=All years # 13 TS=(mother* or maternal*) Indexes=CPCI‐SSH Timespan=All years # 12 TS= (perinatal* or peri‐natal* or prenatal* or pre‐natal* or antenatal* or ante‐natal* or pregnan* or trimester*) Indexes=CPCI‐SSH Timespan=All years # 11 #6 or #10 Indexes=CPCI‐SSH Timespan=All years # 10 #9 OR #8 OR #7 Indexes=CPCI‐SSH Timespan=All years # 9 TS=(Nutributter* or Plumpy* OR LNS or iLiNS) Indexes=CPCI‐SSH Timespan=All years # 8 TS=(lipid* near/3 (supplement* or nutrient* or fortif* or formulation* or enrich* or emuls* or powder* or spread* or paste* )) Indexes=CPCI‐SSH Timespan=All years # 7 TS= ("lipid based") Indexes=CPCI‐SSH Timespan=All years # 6 #1 and #5 Indexes=CPCI‐SSH Timespan=All years # 5 #4 OR #3 OR #2 Indexes=CPCI‐SSH Timespan=All years # 4 TS=("Ready to use" or "point of use" or RUSF or RUTF or (home* near/2 fortif*)) Indexes=CPCI‐SSH Timespan=All years # 3 TS=(complement* near/3 (food* or feed*)) Indexes=CPCI‐SSH Timespan=All years # 2 TS= ((diet* or food*) near/3 (fortif* or enrich* or supplement*)) Indexes=CPCI‐SSH Timespan=All years # 1 TS=(lipid* OR "fatty acid*" OR ((Docosahexaenoic or Eicosapentaenoic) next acid*) or PUFA OR PUFAs OR "omega 3*" OR "omega 6*" OR soy* OR peanut* OR groundnut* OR whey* OR sesame* OR cashew* OR chickpea* OR oil* ) Indexes=CPCI‐SSH Timespan=All years
Cochrane Database of Systematic Reviews (CSDR), part of the Cochrane Library
#1[mh Lipids] #2(fatty next acid*):ti,ab #3((Docosahexaenoic or Eicosapentaenoic) next acid*):ti,ab #4(PUFA or PUFAs):ti,ab #5lipid*:ti,ab #6(omega next (3* or 6*)):ti,ab #7(soy* or peanut or groundnut or whey or sesame or cashew or chickpea or oil*):ti,ab #8{or #1‐#7} #9[mh "Dietary Supplements"] #10[mh "Food, fortified"] #11((diet* or food*) near/3 (fortif* or enrich* or supplement*)):ti,ab #12(complement* near/3 (food* or feed*)):ti,ab #13"Ready to use":ti,ab #14"point of use":ti,ab #15(RUSF or RUTF):ti,ab #16(home* near/2 fortif*):ti,ab #17{or #9‐#16} #18#8 and #17 #19(lipid next based):ti,ab #20(lipid* near/3 supplement*):ti,ab #21(lipid* near/3 nutrient*):ti,ab #22(lipid* near/3 fortif*):ti,ab #23(lipid* near/3 formulation*):ti,ab #24(lipid* near/3 enrich*):ti,ab #25(lipid* near/3 emuls*):ti,ab #26(lipid* near/3 powder*):ti,ab #27(lipid* near/3 spread*):ti,ab #28(lipid* near/3 paste*):ti,ab #29(Nutributter* or Plumpy*):ti,ab #30(LNS* or iLiNS):ti,ab #31{or #19‐#30} #32#18 or #31 #33[mh Pregnancy] #34[mh "pregnant women"] #35[mh "Prenatal care"] #36[mh "Perinatal care"] #37(perinatal* or peri‐natal* or prenatal* or pre‐natal* or antenatal* or ante‐natal*):ti,ab #38pregnan*:ti,ab #39trimester*:ti,ab #40[mh Mothers] #41(mother* or maternal*):ti,ab #42{or #33‐#41} #43#32 and #42 in Cochrane Reviews (Reviews and Protocols)
Database of Abstracts of Reviews of Effect (DARE), part of the Cochrane Library
#1[mh Lipids] #2(fatty next acid*):ti,ab #3((Docosahexaenoic or Eicosapentaenoic) next acid*):ti,ab #4(PUFA or PUFAs):ti,ab #5lipid*:ti,ab #6(omega next (3* or 6*)):ti,ab #7(soy* or peanut or groundnut or whey or sesame or cashew or chickpea or oil*):ti,ab #8{or #1‐#7} #9[mh "Dietary Supplements"] #10[mh "Food, fortified"] #11((diet* or food*) near/3 (fortif* or enrich* or supplement*)):ti,ab #12(complement* near/3 (food* or feed*)):ti,ab #13"Ready to use":ti,ab #14"point of use":ti,ab #15(RUSF or RUTF):ti,ab #16(home* near/2 fortif*):ti,ab #17{or #9‐#16} #18#8 and #17 #19(lipid next based):ti,ab #20(lipid* near/3 supplement*):ti,ab #21(lipid* near/3 nutrient*):ti,ab #22(lipid* near/3 fortif*):ti,ab #23(lipid* near/3 formulation*):ti,ab #24(lipid* near/3 enrich*):ti,ab #25(lipid* near/3 emuls*):ti,ab #26(lipid* near/3 powder*):ti,ab #27(lipid* near/3 spread*):ti,ab #28(lipid* near/3 paste*):ti,ab #29(Nutributter* or Plumpy*):ti,ab #30(LNS* or iLiNS):ti,ab #31{or #19‐#30} #32#18 or #31 #33[mh Pregnancy] #34[mh "pregnant women"] #35[mh "Prenatal care"] #36[mh "Perinatal care"] #37(perinatal* or peri‐natal* or prenatal* or pre‐natal* or antenatal* or ante‐natal*):ti,ab #38pregnan*:ti,ab #39trimester*:ti,ab #40[mh Mothers] #41(mother* or maternal*):ti,ab #42{or #33‐#41} #43#32 and #42 in Other Reviews
Epistemonikos
(epistemonikos.org)
(title:((title:(LIPID* OR FATTY ACID* OR OMEGA OR Docosahexaenoic OR Eicosapentaenoic OR soy* OR peanut OR groundnut OR whey OR sesame OR cashew OR chickpea OR oil*) OR abstract:(LIPID* OR FATTY ACID* OR OMEGA OR Docosahexaenoic OR Eicosapentaenoic OR soy* OR peanut OR groundnut OR whey OR sesame OR cashew OR chickpea OR oil*))) OR abstract:((title:(LIPID* OR FATTY ACID* OR OMEGA OR Docosahexaenoic OR Eicosapentaenoic OR soy* OR peanut OR groundnut OR whey OR sesame OR cashew OR chickpea OR oil*) OR abstract:(LIPID* OR FATTY ACID* OR OMEGA OR Docosahexaenoic OR Eicosapentaenoic OR soy* OR peanut OR groundnut OR whey OR sesame OR cashew OR chickpea OR oil*)))) AND (title:(fortif* OR enrich* OR supplement* OR "Ready to use" OR "point of use" OR RUSF OR RUTF) OR abstract:(fortif* OR enrich* OR supplement* OR "Ready to use" OR "point of use" OR RUSF OR RUTF)) AND (title:(PREGNAN* OR perinatal* OR peri‐natal* OR prenatal* OR pre‐natal* OR antenatal* OR ante‐natal* OR trimester* OR MOTHER* OR MATERNAL*) OR abstract:(PREGNAN* OR perinatal* OR peri‐natal* OR prenatal* OR pre‐natal* OR antenatal* OR ante‐natal* OR trimester* OR MOTHER* OR MATERNAL*)) Limited to Systematic Reviews of Interventions
PoPLINE
((( lipid* OR "fatty acid*" OR PUFA OR PUFAs OR "omega 3*" OR "omega 6*" OR soy* OR peanut* OR groundnut* OR whey* OR sesame* OR cashew* OR chickpea* OR oil* ) AND ( FORTIF* OR ENRICH OR SUPPLEMENT* OR "READY TO USE" OR "POINT OF USE" OR RUSF OR RUTF OR PASTE* OR SPREAD* OR FORMULAT* OR EMULS* OR NUTRIENT* OR POWDER* )) OR ( Nutributter* OR Plumpy* OR LNS OR iLiNS ) ) ) AND (PREGNAN* OR perinatal* OR peri‐natal* OR prenatal* OR pre‐natal* OR antenatal* OR ante‐natal* OR MOTHER* OR MATERNAL*) AND (RANDOM* OR TRIAL* OR CONTROL* OR PLACEBO* OR PROSPECTIV* OR LONGITUDINAL OR BLIND* OR GROUP* OR CLUSTER* OR meta‐analysis OR systematic review)
Clinical Trials
(clinicaltrials.gov)
Interventional Studies CONDITION| pregnancy OR prenatal OR pre‐natal OR antenatal OR ante‐natal OR perinatal OR perinatal OR mothers OR maternal
AND
INTERVENTION| lipid OR LIPID‐BASED OR LNS OR iLiNS OR NUTRIENT SUPPLEMENT OR FORTIFICATION OR fortified OR "READY TO USE" OR "POINT OF USE" OR RUSF OR RUTF OR "THERAPEUTIC FOOD" OR PASTE OR SPREAD OR BLEND OR NUTRIBUTTER OR Plumpy OR PLUMPYNUT
WHO International Clinical Trials Registry Platform (ICTRP)
(apps.who.int/trialsearch)
CONDITION| pregnancy OR prenatal OR pre‐natal OR antenatal OR ante‐natal OR perinatal OR perinatal OR mothers OR maternal
AND
INTERVENTION|lipid OR LIPID‐BASED OR LNS OR iLiNS OR NUTRIENT SUPPLEMENT OR FORTIFICATION OR fortified OR "READY TO USE" OR "POINT OF USE" OR RUSF OR RUTF OR "THERAPEUTIC FOOD" OR PASTE OR SPREAD OR BLEND OR NUTRIBUTTER OR Plumpy OR PLUMPYNUT
AND
RECRUITMENT STATUS|ALL
IBECS (Índice Bibliográfico Español en Ciencias de la Salud)
(ibecs.isciii.es)
WORD| lipid or LIPID‐BASED OR LNS OR iLiNS OR NUTRIENT SUPPLEMENT OR FORTIFICATION OR fortified OR "READY TO USE" OR "POINT OF USE" OR RUSF OR RUTF OR "THERAPEUTIC FOOD" OR PASTE OR SPREAD OR BLEND OR NUTRIBUTTER OR Plumpy OR PLUMPYNUT
AND
WORD| pregnancy OR prenatal OR pre‐natal OR antenatal OR ante‐natal OR perinatal OR perinatal OR mothers OR maternal
SciElo (Scientific Electronic Library Online)
lipid or lipid‐based OR LNS OR iLiNS OR nutrient supplement OR fortification OR fortified OR "ready to use" OR "point of use" OR RUSF OR RUTF OR "therapeutic food" OR paste OR spread OR blend OR nutributter OR Plumpy OR PLUMPYNUT [All indexes]
AND
pregnancy OR prenatal OR pre‐natal OR antenatal OR ante‐natal OR perinatal OR perinatal OR mothers OR maternal [All indexes]
AIM Africa Global Index Medicus (Africa Index Medicus)
(search.bvsalud.org/ghl/?lang=en&submit=Search&where=REGIONAL)
(lipid or lipid‐based OR LNS OR iLiNS OR nutrient supplement OR fortification OR fortified OR "ready to use" OR "point of use" OR RUSF OR RUTF OR "therapeutic food" OR paste OR spread OR blend OR nutributter OR Plumpy OR PLUMPYNUT) AND (pregnancy OR prenatal OR pre‐natal OR antenatal OR ante‐natal OR perinatal OR perinatal OR mothers OR maternal)
IMEMR Global Index Medicus (Index Medicus for the Eastern Mediterranean Region)
(search.bvsalud.org/ghl/?lang=en&submit=Search&where=REGIONAL).
lipid or "lipid‐based" OR LNS OR iLiNS OR "nutrient supplement" OR fortification OR fortified OR "ready to use" OR "point of use" OR RUSF OR RUTF OR "therapeutic food" OR paste OR spread OR blend OR nutributter OR Plumpy OR PLUMPYNUT [KeyWords]
AND
pregnancy OR prenatal OR pre‐natal OR antenatal OR ante‐natal OR perinatal OR perinatal OR mothers OR maternal [KeyWords]
LILACS (Latin American and Caribbean Health Sciences Literature)
(lilacs.bvsalud.org/en)
(tw:(lipid or "lipid‐based" OR LNS OR iLiNS OR "nutrient supplement" OR fortification OR fortified OR "ready to use" OR "point of use" OR RUSF OR RUTF OR "therapeutic food" OR paste OR spread OR blend OR nutributter OR Plumpy OR PLUMPYNUT)) AND (tw:(pregnancy OR prenatal OR pre‐natal OR antenatal OR ante‐natal OR perinatal OR perinatal OR mothers OR maternal))
PAHO/WHO Institutional Repository for Information Sharing
(iris.paho.org/xmlui)
(lipid or lipid‐based OR LNS OR iLiNS OR nutrient supplement OR fortification OR fortified OR "ready to use" OR "point of use" OR RUSF OR RUTF OR "therapeutic food" OR paste OR spread OR blend OR nutributter OR Plumpy OR PLUMPYNUT) AND (pregnancy OR prenatal OR pre‐natal OR antenatal OR ante‐natal OR perinatal OR perinatal OR mothers OR maternal)
WHOLIS Global Index Medicus (WHO Library Database)
(search.bvsalud.org/ghl/?lang=en&submit=Search&where=REGIONAL)
words or phrase "lipid or "lipid‐based" OR LNS OR iLiNS OR "nutrient supplement" OR fortification OR fortified OR "ready to use" OR "point of use" OR RUSF OR RUTF OR "therapeutic food" OR paste OR spread OR blend OR nutributter OR Plumpy OR PLUMPYNUT" AND words or phrase "pregnancy OR prenatal OR pre‐natal OR antenatal OR ante‐natal OR perinatal OR perinatal OR mothers OR maternal"
WPRIM Global Index Medicus (Western Pacific Index Medicus)
(search.bvsalud.org/ghl/?lang=en&submit=Search&where=REGIONAL)
(LNS OR iLiNS OR nutrient supplement OR fortification OR fortified OR ready to use OR point of use OR RUSF OR RUTF OR therapeutic food OR paste OR spread OR blend OR nutributter OR Plumpy OR PLUMPYNUT) AND (pregnancy OR prenatal OR pre‐natal OR antenatal OR ante‐natal OR perinatal OR perinatal OR mothers OR maternal)
IMSEAR Global Index Medicus (Index Medicus for the South‐East Asian Region)
(search.bvsalud.org/ghl/?lang=en&submit=Search&where=REGIONAL)
lipid or "lipid‐based" OR LNS OR iLiNS OR "nutrient supplement" OR fortification OR fortified OR "ready to use" OR "point of use" OR RUSF OR RUTF OR "therapeutic food" OR paste OR spread OR blend OR nutributter OR Plumpy OR PLUMPYNUT [Title]
AND
pregnancy OR prenatal OR pre‐natal OR antenatal OR ante‐natal OR perinatal OR perinatal OR mothers OR maternal [Title]
IndMED
(indmed.nic.in/indmed.html)
(LNS OR iLiNS OR nutrient supplement OR fortification OR fortified OR ready to use OR point of use OR RUSF OR RUTF OR therapeutic food OR paste OR spread OR blend OR nutributter OR Plumpy OR PLUMPYNUT) AND (pregnancy OR prenatal OR pre‐natal OR antenatal OR ante‐natal OR perinatal OR perinatal OR mothers OR maternal)
Native Health Research Database
(hscssl.unm.edu/nhd)
Keywords: (Supplement AND pregnancy)
Appendix 2. Risk of bias
Random sequence generation (checking for possible selection bias)
We described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it produced comparable groups.
Low risk of bias: any truly random process; for example, random number table, computer random number generator.
High risk of bias: any non‐random process; for example, odd or even date of birth, hospital or clinic record number.
Unclear risk of bias: where there was insufficient information provided to permit a judgement of high or low risk of bias.
Allocation concealment (checking for possible selection bias)
We described the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment.
Low risk of bias: for example, telephone or central randomisation; consecutively numbered, sealed, opaque envelopes.
High risk of bias: open random allocation, unsealed or non‐opaque envelopes.
Unclear risk of bias: where there was insufficient information provided to permit a judgement of high or low risk of bias.
Blinding of participants and personnel (checking for possible performance bias)
We described all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received.
Low risk of bias: blinding of participants and personnel and unlikely that blinding could have been broken, or no blinding or incomplete blinding but outcome unlikely to have been influenced.
High risk of bias: participants and personnel not blinded, incomplete or broken blinding, and outcome likely to have been influenced.
Unclear risk of bias: where there was insufficient information provided to permit a judgement of high or low risk of bias.
Whilst we assessed the blinding of participants and personnel separately, we combined the results into a single evaluation of risk of bias associated with blinding.
Blinding of outcome assessment (checking for possible detection bias)
We described all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received.
Low risk of bias: blinding of outcome assessment and unlikely that blinding could have been broken, or no blinding but measurement unlikely to have been influenced.
High risk of bias: for example, no blinding of outcome assessment, where measurement was likely to have been influenced by lack of blinding, or where blinding could have been broken.
Unclear risk of bias: where there was insufficient information provided to permit a judgement of high or low risk of bias.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We assessed the completeness of outcomes in each included study.
Low risk of bias: either there were no missing outcome data or the missing outcome data were unlikely to bias the results based on the following considerations: study authors provided transparent documentation of participant flow throughout the study; the proportion of missing data was similar in the intervention and control groups; the reasons for missing data were provided and balanced across intervention and control groups; or the reasons for missing data were not likely to bias the results (for example, moving house).
High risk of bias: missing outcome data were likely to bias the results based on the following considerations: reasons related to outcome when proportion missing or plausible effect size enough to have a clinically relevant effect; 'as‐treated' analysis with substantial departure from allocation and inappropriate use of imputation (trials also received this rating if an 'as‐treated (per protocol)' analysis was performed with substantial differences between the intervention received and that assigned at randomisation); or if potentially inappropriate methods for imputation were used.
Unclear risk of bias: where there was insufficient information provided to permit a judgement of high or low risk of bias.
Selective reporting (checking for possible reporting bias)
Selective reporting can lead to reporting bias. We compared methods to results and looked for outcomes measured (or likely to have been measured) but not reported.
Low risk of bias: where it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review were reported.
High risk of bias: where not all of the study's prespecified outcomes were reported, one or more reported primary outcomes were not prespecified, outcomes of interest were reported incompletely and so could not be used, or the study failed to include results of a key outcome that was expected to be reported.
Unclear risk of bias: where there was insufficient information provided to permit a judgement of high or low risk of bias.
Other sources of bias (checking for other possible sources of bias)
We assessed if the study was free of other potential bias not covered by the domains above.
Low risk of bias: where there was similarity between outcome measures at baseline, similarity between potential confounding variables at baseline, or adequate protection of study arms against contamination.
High risk of bias: where there was no similarity between outcome measures at baseline, similarity between potential confounding variables at baseline, or adequate protection of study arms against contamination.
Unclear risk of bias: where there was insufficient information provided to permit a judgement of high or low risk of bias.
Data and analyses
Comparison 1. Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gestational weight gain | 2 | 3539 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [‐0.44, 1.36] |
| 2 Maternal anaemia | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Maternal mortality | 3 | 5628 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.12, 2.41] |
| 4 Adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Low birth weight (LBW) | 3 | 4826 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.05] |
| 6 Weight at birth | 3 | 5077 | Mean Difference (IV, Random, 95% CI) | 53.28 [28.22, 78.33] |
| 7 Length at birth | 3 | 4986 | Mean Difference (IV, Random, 95% CI) | 0.24 [0.11, 0.36] |
| 8 Small‐for‐gestational age (SGA) | 3 | 4823 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.89, 0.99] |
| 9 Preterm births | 3 | 4924 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.80, 1.11] |
| 10 Duration of gestation | 3 | 5033 | Mean Difference (IV, Random, 95% CI) | 0.18 [0.04, 0.32] |
| 11 Miscarriage | 2 | 4714 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.66, 1.14] |
| 12 Stillbirth | 3 | 5575 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.52, 2.48] |
| 13 Head circumference | 2 | 4057 | Mean Difference (IV, Random, 95% CI) | 0.20 [0.20, 0.20] |
| 14 Head circumference z score | 3 | 4982 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [0.04, 0.18] |
| 15 Mid‐upper‐arm circumference (MUAC) | 2 | 4374 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.02, 0.26] |
| 16 Newborn stunting | 2 | 4166 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.71, 0.94] |
| 17 Newborn underweight | 2 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.63, 1.13] |
| 18 Neonatal death | 3 | 7172 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.47, 1.10] |
| 18.1 Early neonatal | 3 | 5555 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.45, 1.09] |
| 18.2 Late neonatal | 2 | 1617 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.14, 6.51] |
Comparison 2. Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gestational weight gain per week | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Maternal anaemia | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 LBW | 3 | 2404 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.74, 1.14] |
| 5 Weight at birth | 3 | 2573 | Mean Difference (IV, Random, 95% CI) | 23.67 [‐10.53, 57.86] |
| 6 Length at birth | 3 | 2567 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.02, 0.42] |
| 7 SGA | 3 | 2393 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.07] |
| 8 Preterm births | 3 | 2630 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.93, 1.42] |
| 9 Duration of gestation | 3 | 2740 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.26, 0.12] |
| 10 Head circumference | 2 | 1627 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.16, 0.31] |
| 11 Head circumference z score | 2 | 1549 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.01, 0.21] |
| 12 MUAC | 2 | 1939 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.01, 0.16] |
| 13 Newborn stunting | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 14 Newborn underweight | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 15 Neonatal death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN): subgrouped by energy content.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 LBW | 3 | 2404 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.74, 1.14] |
| 1.1 Small quantity‐lipid‐based nutrient supplements (SQ‐LNS) | 2 | 1384 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.66, 1.20] |
| 1.2 Large quantity‐lipid‐based nutrient supplements (LQ‐LNS) | 1 | 1020 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.69, 1.30] |
| 2 Weight at birth | 3 | 2573 | Mean Difference (IV, Random, 95% CI) | 23.67 [‐10.53, 57.86] |
| 2.1 SQ‐LNS | 2 | 1553 | Mean Difference (IV, Random, 95% CI) | 31.22 [‐12.66, 75.11] |
| 2.2 LQ‐LNS | 1 | 1020 | Mean Difference (IV, Random, 95% CI) | 12.0 [‐42.56, 66.56] |
| 3 Length at birth | 3 | 2567 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.02, 0.42] |
| 3.1 SQ‐LNS | 2 | 1548 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.10, 0.32] |
| 3.2 LQ‐LNS | 1 | 1019 | Mean Difference (IV, Random, 95% CI) | 0.40 [0.09, 0.71] |
| 4 SGA | 3 | 2393 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.07] |
| 4.1 SQ‐LNS | 2 | 1381 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.85, 1.17] |
| 4.2 LQ‐LNS | 1 | 1012 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.76, 1.07] |
| 5 Preterm births | 3 | 2630 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.93, 1.42] |
| 5.1 SQ‐LNS | 2 | 1487 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.74, 1.92] |
| 5.2 LQ‐LNS | 1 | 1143 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.87, 1.51] |
| 6 Duration of gestation | 3 | 2740 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.26, 0.12] |
| 6.1 SQ‐LNS | 2 | 1597 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.23, 0.23] |
| 6.2 LQ‐LNS | 1 | 1143 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.52, 0.12] |
| 7 Head circumference | 2 | 1627 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.16, 0.31] |
| 7.1 SQ‐LNS | 1 | 608 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.01, 0.41] |
| 7.2 LQ‐LNS | 1 | 1019 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.25, 0.17] |
| 8 MUAC | 2 | 1939 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.01, 0.16] |
| 8.1 SQ‐LNS | 1 | 928 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.02, 0.22] |
| 8.2 LQ‐LNS | 1 | 1011 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.07, 0.17] |
| 9 Neonatal death: LQ‐LNS | 1 | 1175 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.36, 2.15] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adu‐Afarwuah 2015.
| Methods | Randomised, single‐blind, parallel group, community‐based clinical trial in Ghana | |
| Participants |
Sample size: 1320 pregnant women and 1228 infants enrolled; 263 pregnant women excluded due to mixed exposure Mean age: 26.6 years Inclusion/exclusion criteria: pregnant women ≥ 18 years of age and ≤ 20 weeks of gestation, and children aged 6 months to 18 months were eligible. Women were excluded if their antenatal cards indicated HIV infection, asthma, epilepsy, tuberculosis, or any malignancy. Additional exclusion criteria were known milk or peanut allergy, not residing in the area, intention to move within the next 2 years, unwillingness to receive field workers or take the study supplement, or participation in another trial. |
|
| Interventions |
Interventions Pregnant women were allocated to 3 groups:
Delivery/administration At enrolment, each woman received a 2‐week supply of supplement (including instruction to consume one capsule with water after a meal or one sachet mixed with any food each day), a standard nutrition message (quote: "Do not forget to eat meat, fish, eggs, fruits, and vegetables whenever you can; you still need these foods even as you take the supplement we have given you"), and a sticker on her antenatal card for identification. LNS composition Ration: 20 g/d Total energy: 118 kcal Protein: 2.6 g Fat: 10 g Linoleic acid: 4.59 g α‐Linolenic acid: 0.59 g Vitamin A: 800 µg RE Vitamin C: 100 mg Vitamin B1: 2.8 mg Vitamin B2: 2.8 mg Niacin: 36 mg Folic acid: 400 µg Pantothenic acid: 77 mg Vitamin B6: 3.8 mg Vitamin B12: 5.2 µg Vitamin D: 400 IU Vitamin E: 20 mg Vitamin K: 45 µg Iron: 20 mg Zinc: 30 mg Copper: 4 mg Calcium: 280 mg Phosphorus: 190 mg Potassium: 200 mg Magnesium: 65 mg Selenium: 130 µg Iodine: 250 µg Manganese: 2.6 mg |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Study duration: December 2009 to March 2014 Conflict of interest: authors declared that they had no conflicts of interest related to this study. Source of funding: Bill & Melinda Gates Foundation. The funder of the study had no role in the study design; data collection, analysis, and interpretation; or the preparation of the manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "The study statistician at University of California, Davis developed group allocations with the use of a computer‐generated (SAS version 9.3; SAS Institute) randomisation scheme in blocks of 9" Comment: adequately done |
| Allocation concealment (selection bias) | Low risk |
Quote: "The study nurse offered sealed, opaque envelopes bearing group allocations, 9 envelopes at a time, and the woman picked one to reveal the allocation. Allocation information was kept securely by the field supervisor and the study statistician only." Comment: adequately done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it was not possible to blind the field workers and study participants to those consuming capsules versus LNS (because of the starkly different characteristics). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "Laboratory staff, anthropometrists, and data analysts had no knowledge of group assignment until all preliminary analyses had been completed" Comment: adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: in IFA arm: 15/408 (3.7%) lost to follow‐up; in MMN arm: 10/411 (2.4%) lost to follow‐up; in LNS arm: 18/409 (4.4%) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: registered at ClinicalTrials.gov as NCT00970866. Statistical analysis plan was posted before (www.ilins.org) and all presented analyses were prespecified in the statistical analysis plan; adequately done |
| Other bias | Low risk | Comment: no other sources of bias were identified. The study was funded by the Bill & Melinda Gates Foundation. |
Ashorn 2015.
| Methods | A randomised, outcome‐assessor‐blinded, community‐based clinical trial in Malawi | |
| Participants |
Sample size: 1391 pregnant women enrolled; 84 women lost to follow‐up Mean age: 25 years Inclusion/exclusion criteria: eligible women had an ultrasound confirmed pregnancy of no more than 20 completed gestation weeks, residence in the defined catchment area, availability during the period of the study, and signed or thumb‐printed informed consent. The study excluded those: younger than 15 years of age; in need for frequent medical attention due to a chronic health condition; diagnosed asthma treated with regular medication; severe illness warranting hospital referral; history of peanut allergy; history of anaphylaxis or serious allergic reaction to any substance; requiring emergency medical care; pregnancy complications evident at enrolment visit (moderate to severe oedema, blood haemoglobin concentration 50 g/L, systolic blood pressure of 160 mmHg or more, or diastolic blood pressure 100 mmHg or more); earlier participation in the iLiNS‐DYAD‐M trial (during a previous pregnancy); or concurrent participation in any other clinical trial. |
|
| Interventions |
Interventions Pregnant women were allocated to 3 groups
Delivery/administration Data collectors made home visits, biweekly, to deliver the supplements and to collect information on the participant's adherence to the study intervention. LNS composition Total energy: 118 kcal Protein: 2.6 g Fat: 10 g Linoleic acid: 4.59 g α‐Linolenic acid: 0.59 g Vitamin A: 800 mg RE Vitamin C: 100 mg Vitamin B1: 2.8 mg Vitamin B2: 2.8 mg Niacin: 36 mg Folic acid: 400 mg Pantothenic acid: 7 mg Vitamin B6: 3.8 mg Vitamin B12: 5.2 mg Vitamin D: 10 mg Vitamin E: 20 mg Vitamin K: 45 mg Iron: 20 mg Zinc: 30 mg Copper: 4 mg Calcium: 280 mg Phosphorus: 190 mg Potassium: 200 mg Magnesium: 65 mg Selenium: 130 mg Iodine: 250 mg Manganese: 2.6 mg |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Study duration: February 2011 to December 2015 Conflict of interest: one of the authors, Mamane Zeilani, works as a Drector of Research for Nutriset SAS, a company that produces and sells LNS supplements and also prepared the LNS supplements purchased for the current trial. The other authors declared no conflicts of interest related to this study. Source of funding: supported, in part, by a grant from the Bill & Melinda Gates Foundation, with additional funding from the Office of Health, Infectious Diseases, and Nutrition, Bureau for Global Health, US Agency for International Development (USAID) under terms of Cooperative Agreement No. AID‐OAA‐A‐12‐00005, through the Food and Nutrition Technical Assistance III Project (FANTA), managed by FHI 360. For data management and statistical analysis, the team received additional support in grants from the Academy of Finland (grant 252075) and the Medical Research Fund of Tampere University Hospital (grant 9M004). The author Yin Bun Cheung was supported by the Singapore Ministry of Health's National Medical Research Council under its Clinician Scientist Award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "A study statistician not involved in data collection generated 4 randomization code lists in blocks of 9." Comment: adequately done |
| Allocation concealment (selection bias) | Low risk |
Quote: "In the randomisation process, each participant number was allocated one of 9 possible letter codes (A, B, C, D,E, H, J, K, or M). Each letter code corresponded to one of the 3 interventions (i.e., each intervention matched with 3 separate letter codes)." Comment: adequately done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Quote: "The IFA and MMN interventions were provided by using double‐masked procedures—that is, the capsules looked identical,and neither the participants nor the research team members were aware of the nutrient contents of the supplement capsules. For the LNS group, we used single‐masked procedures—that is field workers who delivered the supplements knew which mothers were receiving LNS (but not a difference between IFA and MMN), and the participants were advised not to disclose information about their supplements to anyone other than an iLiNS team member." Comment: it was not possible to blind field workers due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "The data collectors who performed the anthropometric measurements or assessed other outcomes were not aware of group allocation." Comment: adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: overall 6.04% similar between groups; adequately done |
| Selective reporting (reporting bias) | Low risk | Comment: all presented analyses were prespecified either in the trial protocol or in the statistical analysis plan. Registered at clinicaltrials.gov as NCT01239693. Adequately done |
| Other bias | Low risk | Comment: no other sources of bias were identified. The study was supported, in part, by a grant to the University of California, Davis, from the Bill & Melinda Gates Foundation, with additional funding from the Office of Health, Infectious Diseases, and Nutrition, Bureau for Global Health, US Agency for International Development (USAID). |
Huybregts 2009 (C).
| Methods | A randomised, open‐label, community‐based clinical trial in the rural settings of Burkina Faso | |
| Participants |
Sample size: 1296 pregnant women enrolled; 32 women lost to follow‐up Mean age: 24.5 years Inclusion/exclusion criteria: women with suspected pregnancy were referred to the health centre for a formal pregnancy test and were included after confirmation of pregnancy. No exclusion criteria were used except that participants planning to leave the study area within the next 2 years were not eligible. |
|
| Interventions |
Interventions Pregnant women were allocated to 2 groups:
Delivery/administration Home visitors kept both the MMN and fortified food supplement with them and visited 10 to 20 participants per day to provide and directly observe the supplement intake. LNS composition Energy: 1.56 MJ Energy from protein: 15.8% Energy from fat: 67.05% Carbohydrates: 15.9 g Protein: 14.7 g Fat: 27.6 g SFA: 8,1 g MUFA: 12.1 g PUFA: 7.3 g x3 fatty acids: 0.4 g x6 fatty acids: 7.0 g Total dietary fiber: 9.1 g Vitamin A: 881 RE Vitamin D: 200 IU Vitamin E: 13 mg Thiamin: 1.6 mg Riboflavin: 1.6 mg Niacin: 21 mg Vitamin B6: 2.0 mg Folate: 461 µg Vitamin B12: 2.6 µg Vitamin C: 71 mg Zinc: 17 mg Iron: 35 mg Copper: 2.7 mg Selenium: 65 µg Iodine: 150 µg Calcium: 90 mg |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Study duration: March 2006 to June 2008 Conflict of interest: authors declared that none of the authors had any potential conflicts of interest. Source of funding: Supported by Nutrition Third World, the Belgian Ministry of Development under agreement 96501, and the Flemish University Council under contract ZEIN2004PR298. None of the funders had any role in the study design, data collection, data analysis, or report writing. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Individual randomisation was performed based on a computer‐generated program in permuted blocks of 4" Comment: adequately done |
| Allocation concealment (selection bias) | Low risk |
Quote: "Randomization numbers were sealed in opaque envelopes and when an informed consent was obtained from an eligible participant, the study physician opened the next envelope and assigned the participant to a treatment group" Comment: adequately done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Quote: "We conducted a nonblinded, individually randomized controlled trial." Comment: not clear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Quote: "The study was organized as an open‐label" Comment: not clear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 150/641 (23%) lost to follow‐up in group 1; 164/655 (25%) lost to follow‐up in group 2 |
| Selective reporting (reporting bias) | Low risk | Comment: all presented analyses were prespecified either in the trial protocol or in the statistical analysis plan registered at Clinical.Trials.gov as NCT00909974 |
| Other bias | Low risk | Comment: no other sources of bias were identified. The study was supported by the Flemish University Council, Nutrition Third World, and the Belgian Ministry of Development. The multiple micronutrient premix was donated by Nutriset, France. |
Mridha 2016b.
| Methods | A cluster‐randomised, community‐based trial in Bangladesh | |
| Participants |
Sample size: 4011 pregnant women ≤ 20 weeks gestation enrolled; 93 women lost to follow‐up Mean age: 21.9 years Inclusion/exclusion criteria: the eligibility criteria included gestational age < 20 weeks and no plans to move out of the study area during pregnancy or the following 3 years (i.e. a permanent resident of the study area) |
|
| Interventions |
Interventions Pregnant women were allocated to 2 groups:
Delivery/administration: The first 1‐month supply of supplements for each woman enrolled was delivered by the community health worker at the safe delivery unit right after the baseline data were collected by the evaluation staff. Subsequent monthly supplies were usually delivered by the community health worker or village health volunteer to the woman's home, but occasionally delivery occurred during educational sessions given by the community health worker or village health volunteer. LNS composition Ration: 20 g/d Total energy: 118 kcal Protein: 2.6 g Fat: 10 g Linoleic acid: 4.59 g α‐Linolenic acid: 0.59 g Vitamin A: 800 µg RE Vitamin C: 100 mg Thiamin: 2.8 mg Riboflavin: 2.8 mg Niacin: 36 mg Folic acid: 400 µg Pantothenic acid: 7 mg Vitamin B6: 3.8 mg Vitamin B12: 5.2 µg Vitamin D: 400 IU Vitamin E: 20 mg Vitamin K: 45 µg Iron: 20 mg Zinc: 30 mg Copper: 4 mg Calcium: 280 mg Phosphorus: 190 mg Potassium: 200 mg Magnesium: 65 mg Selenium: 130 µg Iodine: 250 µg Manganese: 2.6 mg |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Study duration: October 2011 to August 2012 Conflict of interest: the authors declared that they had no conflicts of interest. Source of funding: US Agency for International Development's Food and Nutrition Technical Assistance III Project (FANTA), managed by Family Health International 360 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "The study statistician at UC Davis first stratified the 64 clusters by sub district and union, and then assigned each cluster to 1 of 4 sets containing 16 clusters each. This procedure was then replicated several thousand times, and each randomisation was tested for balance across groups with respect to mean cluster population, number of clinics and health workers per 1000 people, number of health‐/nutrition‐related nongovernmental organizations in the cluster, and the source of funding for the CHDP, as well as the SD of the cluster population size" Comment: adequately done |
| Allocation concealment (selection bias) | Low risk |
Quote: "The final randomisation to the 4 arms was then chosen at random from the acceptable potential randomizations; and the letters A, B, C, and D were assigned to the 4 sets, randomly permuting them by sorting on a randomly generated, uniformly distributed number (with the use of SAS for Windows, release 9.2; SAS Institute)" Comment: adequately done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it was not possible to blind the study participants to those consuming capsules versus LNS (because of the starkly different characteristics). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "The evaluation staff was not involved in supplement delivery" Comment: adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 413/2964 (13.9%) lost to follow‐up in group 1; 149/1047 (14.23%) lost to follow‐up in group 2 |
| Selective reporting (reporting bias) | Low risk | Comment: all presented analyses were prespecified either in the trial protocol or in the statistical analysis plan registered at Clinical.Trials.gov as clinicaltrials.gov/ct2/show/NCT01715038; adequately done |
| Other bias | Low risk | Comment: no other sources of bias identified. The study was carried out by 3 partners: LAMB , the International Center for Diarrheal Disease Research, Bangladesh (ICDDR,B); and the University of California, Davis (UC Davis). |
BMI: body mass index; CHDP: Child Health and Disability Prevention; HCZ: head‐circumference‐for‐age z score; IFA: iron and folic acid; iLiNS: International LIpid‐based Nutrient Supplement; iLiNS‐DYAD‐M trial: a trial conducted by the iLiNS study group, which enrolled mother‐child dyads in Malawi; IU: international units; LAZ: length‐for‐age z score; LNS: lipid‐based nutrient supplements; MMN: multiple micronutrients; MUAC: mid‐upper‐arm circumference; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; RE: retinol equivalent; SAS: suite of analytics software; SD: standard deviation; SFA: saturated fatty acid; UC: University of California; WAZ: weight‐for‐age z score; WLZ: weight‐for‐length z score.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adams 2017 | Reports longitudinal, household food‐insecurity data from four studies |
| Adams 2018 | Assesses willingness to pay for small‐quantity, LNS for women and children from studies conducted in Ghana and Malawi |
| Flax 2012 | Includes HIV‐infected women |
| Flax 2014 | Includes HIV‐infected women |
| Haber 2016 | LNS given to women in postpartum period to improve quality of breast milk |
| Hampel 2018 | Assesses effects of an LNS and antiretroviral therapy on iron, copper and zinc in milk from HIV‐infected Malawian mothers |
| Johnson 2017 | LNS not tested as a separate arm but with protein energy supplementation |
| Kayira 2012 | Includes HIV‐infected women |
| Oaks 2017 | A sub‐study of two included studies evaluating the effects of LNS on maternal plasma fatty acid status and lipid profile |
| Prado 2017 | Study to determine factors associated with 18‐month language and motor development in 4 prospective cohorts of children who participated in studies conducted as part of the International Lipid‐Based Nutrient Supplements (iLiNS) Project in Ghana, Malawi and Burkina Faso |
| Schlossman 2017 | LNS provided to mothers with no regard to pregnancy status |
| Young 2015 | Formative research related to acceptability of LNS by women |
LNS: lipid‐based nutrient supplements.
Characteristics of ongoing studies [ordered by study ID]
Fernald 2016.
| Trial name or title | A cluster‐randomized, controlled trial of nutritional supplementation and promotion of responsive parenting in Madagascar: the MAHAY ["smart" in Malagasy] study design and rationale |
| Methods | A cluster‐randomised, multi‐arm, controlled trial |
| Participants |
Number of clusters: 25 communities, 5 per arm Sample size: 1250 pregnant women; 1250 children aged 0‐6 months old; 1250 children aged 6‐18 months Inclusion/exclusion criteria: all pregnant women and women with age‐eligible children living in the catchment area of a project site are eligible to participate in the standard growth monitoring and nutritional education that occurs in a group setting in a community centre in all sites. |
| Interventions |
Interventions Participants will be allocated to 5 groups (each group will comprise 1250 pregnant women, 1250 children 0‐6 months old, and1250 children 6‐18 months old);
For this review, we will include groups T3 as intervention arm and T0 as control arm. |
| Outcomes | The following outcomes will be measured at baseline, 1 year and 2 years after the baseline survey. Primary outcomes
Secondary outcomes
|
| Starting date | July 2014 to December 2019 |
| Contact information |
Name: Professor Lia Fernald Email: fernald@berkeley.edu |
| Notes | This study is funded by Strategic Impact Evaluation Fund (SIEF), the World Bank Innovation Grant, the Early Learning Partnership Grant, and the National Nutrition Office in Madagascar. |
LNS: lipid‐based supplementation.
Differences between protocol and review
-
We added a separate comparison for 'LNS versus IFA'; in our protocol, Das 2017, we planned to treat this comparison under 'LNS versus no intervention or placebo'.
-
We did not include 'maternal mortality' and 'duration of gestation' in our list of outcomes in our protocol (Das 2017). However, as three studies reported on these outcomes, we decided to include 'maternal mortality' as a primary outcome and 'duration of gestation' as a secondary outcome in the review.
Contributions of authors
All authors contributed to the development of the review. Rehana A Salam (RAS), Zahra Hoodbhoy (ZH), Afsah Zulfiqar Bhutta (AZB) and Nancy G Valenzuela‐Rubio (NV) selected which studies to include, obtained copies of the studies, and extracted data from studies. Jai K Das (JKD) and RAS entered data into RevMan 5 (Review Manager 2014), carried out and interpreted the analyses. Zita Weise Prinzo (ZWP), Zulfiqar A Bhutta (ZAB), JKD, RAS and ZH drafted the final review.
As the contact author, Jai K Das has overall responsibility for the review.
Sources of support
Internal sources
-
Aga Khan University, Karachi, Pakistan.
Employer of JKD, RAS and ZH.
-
Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, World Health Organization (WHO), Switzerland.
ZWP is a member of staff at the Department of Nutrition for Health and Development at the WHO.
External sources
-
The Bill & Melinda Gates Foundation, USA.
WHO acknowledges the financial support from The Bill & Melinda Gates Foundation, for the development of systematic reviews of the evidence on the effects of nutrition interventions.
Declarations of interest
Jai K Das ‐ none known. Zahra Hoodbhoy ‐ none known. Rehana A Salam ‐ none known. Afsah Zulfiqar Bhutta ‐ none known. Nancy G Valenzuela‐Rubio ‐ none known. Zita Weise Prinzo is a full‐time staff member of the World Health Organization (WHO). Zulfiqar A Bhutta's institution received a grant from the WHO to undertake this review.
Disclaimer: Zita Weise Prinzo is a full‐time staff member of the WHO. The author alone is responsible for the views expressed in this publication; the views do not necessarily represent the official position, decisions, policy or views of the WHO.
New
References
References to studies included in this review
Adu‐Afarwuah 2015 {published data only}
- Adams KP, Lybbert TJ, Vosti SA, Ayifah E, Arimond M, Adu‐Afarwuah S, et al. Unintended effects of a targeted maternal and child nutrition intervention on household expenditures, labor income, and the nutritional status of non‐targeted siblings in Ghana. World Development 2018;107:138‐50. [DOI: 10.1016/j.worlddev.2018.02.025; NCT00970866] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams KP, Okronipa H, Adu‐Afarwuah S, Arimond M, Kumordzie S, Oaks BM, et al. Ghanaian parents' perceptions of pre and postnatal nutrient supplements and their effects. Maternal & Child Nutrition 2018;15:e12608. [DOI: 10.1111/mcn.12608; NCT00970866] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu‐Afarwuah S, Lartey A, Okronipa H, Ashorn P, Arimond M, Dewey KG. Prenatal supplementation with small quantity lipid based nutrient supplements or multiple micronutrients increase urinary iodine concentration in semi‐urban Ghana: a randomized controlled trial. Annals of Nutrition and Metabolism 2017;71(Suppl 2):309. [DOI: 10.1159/000480486; EMBASE: 619277238; NCT00970866; PUBMED: 29024932] [DOI] [PubMed] [Google Scholar]
- Adu‐Afarwuah S, Lartey A, Okronipa H, Ashorn P, Ashorn U, Zeilani M, et al. Maternal supplementation with small‐quantity lipid‐based nutrient supplements compared with multiple micronutrients, but not with iron and folic acid, reduces the prevalence of low gestational weight gain in semi‐urban Ghana: a randomized controlled trial. Journal of Nutrition 2017;147(4):697‐705. [DOI: 10.3945/jn.116.242909; NCT00970866; PMC5368579; PUBMED: 28275100] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu‐Afarwuah S, Lartey A, Okronipa H, Ashorn P, Peerson JM, Arimond M, et al. Small‐quantity, lipid‐based nutrient supplements provided to women during pregnancy and 6 mo postpartum and to their infants from 6 mo of age increase the mean attained length of 18‐mo‐old children in semi‐urban Ghana: a randomized controlled trial. American Journal of Clinical Nutrition 2016;104(3):797‐808. [DOI: 10.3945/ajcn.116.134692; NCT00970866; PMC4997301; PUBMED: 27534634] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu‐Afarwuah S, Lartey A, Okronipa H, Ashorn P, Zeilani M, Baldiviez LM, et al. Impact of small‐quantity lipid‐based nutrient supplement on hemoglobin, iron status and biomarkers of inflammation in pregnant Ghanaian women. Maternal & Child Nutrition 2017;13(2):e12262. [DOI: 10.1111/mcn.12262; NCT00970866; PUBMED: 26924599] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu‐Afarwuah S, Lartey A, Okronipa H, Ashorn P, Zeilani M, Peerson JM, et al. Lipid‐based nutrient supplement increases the birth size of infants of primiparous women in Ghana. American Journal of Clinical Nutrition 2015;101(4):835‐46. [DOI: 10.3945/ajcn.114.091546; NCT00970866.; PUBMED: 25833980] [DOI] [PubMed] [Google Scholar]
- Adu‐Afarwuah S, Lartey A, Zeilani M, Dewey KG. Acceptability of lipid‐based nutrient supplements (LNS) among Ghanaian infants and pregnant or lactating women. Maternal & Child Nutrition 2011;7(4):344‐56. [DOI: 10.1111/j.1740-8709.2010.00286.x; NCT00970866; PUBMED: 21496207] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevor MK, Haskell MJ, Lartey A, Adu‐Afarwuah S, Zeilani M, Dewey KG. Lipid‐based nutrient supplements providing approximately the recommended daily intake of vitamin A do not increase breast milk retinol concentrations among Ghanaian women. Journal of Nutrition 2016;146(2):335‐42. [DOI: 10.3945/jn.115.217786; NCT00970866; PUBMED: 26740682] [DOI] [PubMed] [Google Scholar]
- Oaks BM, Laugero KD, Stewart CP, Adu‐Afarwuah S, Lartey A, Ashorn P, et al. Late‐pregnancy salivary cortisol concentrations of Ghanaian women participating in a randomized controlled trial of prenatal lipid‐based nutrient supplements. Journal of Nutrition 2016;146(2):343‐52. [DOI: 10.3945/jn.115.219576; NCT00970866; PUBMED: 26764321] [DOI] [PubMed] [Google Scholar]
- Okronipa H, Adu‐Afarwuah S, Lartey A, Ashorn P, Vosti SA, Young RR, et al. Maternal supplementation with small‐quantity lipid‐based nutrient supplements during pregnancy and lactation does not reduce depressive symptoms at 6 months postpartum in Ghanaian women: a randomized controlled trial. Archives of Women's Mental Health 2018;21(1):55‐63. [DOI: 10.1007/s00737-017-0752-7; NCT00970866; PMC5762799; PUBMED: 28698916] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okronipa H, Adu‐Afarwuah S, Lartey A, Ashorn P, Vosti SA, Young RR, et al. Maternal supplementation with small‐quantity lipid‐based nutrient supplements during pregnancy and lactation does not reduce depressive symptoms at 6 months postpartum in Ghanaian women: a randomized controlled trial. Archives of Women's Mental Health 2018;21(1):55‐63. [DOI: 10.1007/s00737-017-0752-7; NCT00970866; PMC5762799; PUBMED: 28698916] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okronipa HET, Adu‐Afarwuah S, Lartey A, Ashorn P, Vosti SA, Young RT, et al. The impact of lipid‐based nutrient supplements on maternal depression at 6 mo postpartum in Ghana: a randomized‐controlled trial. FASEB Journal 2016;30(Suppl 1):1172.11. [DOI: 10.1096/fasebj.30.1_supplement.1172.11] [DOI] [Google Scholar]
- Prado EL, Adu‐Afarwuah S, Lartey A, Ocansey M, Ashorn P, Vosti SA, et al. Effects of pre‐and post‐natal lipid‐based nutrient supplements on infant development in a randomized trial in Ghana. Early Human Development 2016;99:43‐51. [DOI: 10.1016/j.earlhumdev.2016.05.011; NCT00970866; PUBMED: 27391572] [DOI] [PubMed] [Google Scholar]
Ashorn 2015 {published data only}
- Ashorn P, Alho L, Ashorn U, Cheung YB, Dewey KG, Gondwe A, et al. Supplementation of maternal diets during pregnancy and for 6 months postpartum and infant diets thereafter with small‐quantity lipid‐based nutrient supplements does not promote child growth by 18 months of age in rural Malawi: a randomized controlled trial. Journal of Nutrition 2015;145(6):1345‐53. [DOI: 10.3945/jn.114.207225; NCT01239693; PUBMED: 25926413] [DOI] [PubMed] [Google Scholar]
- Ashorn P, Alho L, Ashorn U, Cheung YB, Dewey KG, Harjunmaa U, et al. The impact of lipid‐based nutrient supplement provision to pregnant women on newborn size in rural Malawi: a randomized controlled trial. American Journal of Clinical Nutrition 2015;101(2):387‐97. [DOI: 10.3945/ajcn.114.088617; NCT01239693; PUBMED: 25646337] [DOI] [PubMed] [Google Scholar]
- Chandrasiri UP, Fowkes FJ, Richards JS, Langer C, Fan YM, Taylor SM, et al. The impact of lipid‐based nutrient supplementation on anti‐malarial antibodies in pregnant women in a randomized controlled trial. Malaria Journal 2015;14:193. [DOI: 10.1186/s12936-015-0707-2; NCT01239693; PMC4438573; PUBMED: 25957793] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JM, Arnold C, Ashorn P, Ashorn U, Chaima D, Cheung YB, et al. Lipid‐based nutrient supplements during pregnancy and lactation did not affect human milk oligosaccharides and bioactive proteins in a randomized trial. Journal of Nutrition 2017;147(10):1867‐74. [DOI: 10.3945/jn.117.252981; EMBASE: 618612500; NCT01239693; PMC5610548; PUBMED: 28794206] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevor MK, Adu‐Afarwuah S, Ashorn P, Arimond M, Dewey KG, Lartey A, et al. A mixed method study exploring adherence to and acceptability of small quantity lipid‐based nutrient supplements (SQ‐LNS) among pregnant and lactating women in Ghana and Malawi. BMC Pregnancy & Childbirth 2016;16:253. [DOI: 10.1186/s12884-016-1039-0; NCT01239693; PMC5004276; PUBMED: 27577112] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkhoma M, Ashorn P, Ashorn U, Dewey KG, Gondwe A, Mbotwa J, et al. Providing lipid‐based nutrient supplement during pregnancy does not reduce the risk of maternal P falciparum parasitaemia and reproductive tract infections: a randomised controlled trial. BMC Pregnancy & Childbirth 2017;17:35. [DOI: 10.1186/s12884-016-1215-2; NCT01239693; PMC5240436; PUBMED: 28095801] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkhoma M, Ashorn P, Ashorn U, Dewey KG, Gondwe A, Mbotwa J, et al. Providing lipid‐based nutrient supplement during pregnancy does not reduce the risk of maternal P falciparum parasitaemia and reproductive tract infections: a randomised controlled trial. BMC Pregnancy & Childbirth 2017;17:35. [DOI: 10.1186/s12884-016-1215-2; NCT01239693; PMC5240436; PUBMED: 28095801] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado EL, Ashorn U, Phuka J, Maleta K, Sadalaki J, Oaks BM, et al. Associations of maternal nutrition during pregnancy and post‐partum with maternal cognition and caregiving. Maternal & Child Nutrition 2018;14(2):e12546. [DOI: 10.1111/mcn.12546; NCT01239693; PMC5901033; PUBMED: 29098783] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado EL, Maleta K, Ashorn P, Ashorn U, Vosti SA, Sadalaki J, et al. Effects of maternal and child lipid‐based nutrient supplements on infant development: a randomized trial in Malawi. American Journal of Clinical Nutrition 2016;103(3):784‐93. [DOI: 10.3945/ajcn.115.114579; NCT01239693; PUBMED: 26843155] [DOI] [PubMed] [Google Scholar]
- Stewart CP, Oaks BM, Laugero KD, Ashorn U, Harjunmaa U, Kumwenda C, et al. Maternal cortisol and stress are associated with birth outcomes, but are not affected by lipid‐based nutrient supplements during pregnancy: an analysis of data from a randomized controlled trial in rural Malawi. BMC Pregnancy & Childbirth 2015;15:346. [DOI: 10.1186/s12884-015-0793-8; NCT01239693; PMC4688934; PUBMED: 26694646] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RC, Ashorn P, Umar E, Dewey KG, Ashorn U, Creed F, et al. The impact of maternal diet fortification with lipid‐based nutrient supplements on postpartum depression in rural Malawi: a randomised‐controlled trial. Maternal & Child Nutrition 2017;13(2):e12299. [DOI: 10.1111/mcn.12299; NCT01239693; PUBMED: 27060705] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RC, Ashorn P, Umar E, Dewey KG, Ashorn U, Creed F, et al. The impact of maternal diet fortification with lipid‐based nutrient supplements on postpartum depression in rural Malawi: a randomised‐controlled trial. Maternal & Child Nutrition 2017;13(2):e12299. [DOI: 10.1111/mcn.12299; NCT01239693; PUBMED: 27060705] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huybregts 2009 (C) {published data only}
- Huybregts L, Roberfroid D, Lanou H, Meda N, Taes Y, Valea I, et al. Prenatal lipid‐based nutrient supplements increase cord leptin concentration in pregnant women from rural Burkina Faso. Journal of Nutrition 2013;143(5):576‐83. [DOI: 10.3945/jn.112.171181; NCT00909974; PUBMED: 23535609] [DOI] [PubMed] [Google Scholar]
- Huybregts L, Roberfroid D, Lanou H, Menten J, Meda N, Camp J, et al. Prenatal food supplementation fortified with multiple micronutrients increases birth length: a randomized controlled trial in rural Burkina Faso. American Journal of Clinical Nutrition 2009;90(6):1593‐600. [DOI: 10.3945/ajcn.2009.28253; NCT00909974; PUBMED: 19812173] [DOI] [PubMed] [Google Scholar]
- Lanou H, Huybregts L, Roberfroid D, Nikièma L, Kouanda S, Camp J, et al. Prenatal nutrient supplementation and postnatal growth in a developing nation: an RCT. Pediatrics 2014;133(4):e1001‐8. [DOI: 10.1542/peds.2013-2850; NCT00909974; PUBMED: 24590752] [DOI] [PubMed] [Google Scholar]
- Toe LC, Bouckaert KP, Beuf K, Roberfroid D, Meda N, Thas O, et al. Seasonality modifies the effect of a lipid‐based nutrient supplement for pregnant rural women on birth length. Journal of Nutrition 2015;145(3):643‐9. [DOI: 10.3945/jn.114.203448; NCT00909974; PUBMED: 25733482] [DOI] [PubMed] [Google Scholar]
Mridha 2016b {published data only}
- Dewey KG, Mridha MK, Matias SL, Arnold CD, Cummins JR, Khan MS, et al. Lipid‐based nutrient supplementation in the first 1000 d improves child growth in Bangladesh: a cluster‐randomized effectiveness trial. American Journal of Clinical Nutrition 2017;105(4):944‐57. [DOI: 10.3945/ajcn.116.147942; NCT01715038; PUBMED: 28275125] [DOI] [PubMed] [Google Scholar]
- Harding KL, Matias SL, Mridha MK, Moniruzzaman M, Vosti SA, Hussain S, et al. Adherence to recommendations on lipid‐based nutrient supplement and iron and folic acid tablet consumption among pregnant and lactating women participating in a community health programme in northwest Bangladesh. Maternal & Child Nutrition 2017;13(1):e12252. [DOI: 10.1111/mcn.12252; NCT01715038; PUBMED: 26898720] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias SL, Mridha MK, Paul RR, Hussain S, Vosti SA, Arnold CD, et al. Prenatal lipid‐based nutrient supplements affect maternal anthropometric indicators only in certain subgroups of rural Bangladeshi women. Journal of Nutrition 2016;146(9):1775‐82. [DOI: 10.3945/jn.116.232181; NCT01715038; PUBMED: 27440259] [DOI] [PubMed] [Google Scholar]
- Matias SL, Mridha MK, Tofail F, Arnold CD, Khan MS, Siddiqui Z, et al. Home fortification during the first 1000 d improves child development in Bangladesh: a cluster‐randomized effectiveness trial. American Journal of Clinical Nutrition 2017;105(4):958‐69. [DOI: 10.3945/ajcn.116.150318; NCT01715038; PUBMED: 28275128] [DOI] [PubMed] [Google Scholar]
- Mridha MK, Matias SL, Chaparro CM, Paul RR, Hussain S, Vosti SA, et al. Lipid‐based nutrient supplements for pregnant women reduce newborn stunting in a cluster‐randomized controlled effectiveness trial in Bangladesh. American Journal of Clinical Nutrition 2016;103(1):236‐49. [DOI: 10.3945/ajcn.115.111336; NCT01715038; PUBMED: 26607935] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mridha MK, Matias SL, Khan SA, Paul RR, Siddiqui Z, Ullah B, et al. High prevalence of low urinary iodine among pregnant and lactating women of Bangladesh does not respond to daily lipid‐based nutrient supplement containing 250 μg iodine. FASEB Journal 2016;30(1 Suppl):150.4. [www.fasebj.org/doi/abs/10.1096/fasebj.30.1_supplement.150.4] [Google Scholar]
- Mridha MK, Matias SL, Paul RR, Hussain S, Khan MS, Siddiqui Z, et al. Daily consumption of lipid‐based nutrient supplements containing 250 μg iodine does not increase urinary iodine concentrations in pregnant and postpartum women in Bangladesh. Journal of Nutrition 2017;147(8):1586‐92. [DOI: 10.3945/jn.117.248963; NCT01715038; PUBMED: 28615379] [DOI] [PubMed] [Google Scholar]
- Mridha MK, Matias SL, Paul RR, Hussain S, Sarker M, Hossain M, et al. Prenatal lipid‐based nutrient supplements do not affect pregnancy or childbirth complications or cesarean delivery in Bangladesh: a cluster‐randomized controlled effectiveness trial. Journal of Nutrition 2017;147(9):1776‐84. [DOI: 10.3945/jn.117.248880; NCT01715038; PUBMED: 28724657] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adams 2017 {published data only}
- Adams KP, Ayifah E, Phiri TE, Mridha MK, Adu‐Afarwuah S, Arimond M, et al. Maternal and child supplementation with lipid‐based nutrient supplements, but not child supplementation alone, decreases self‐reported household food Insecurity in some settings. Journal of Nutrition 2017;147(12):2309‐18. [DOI: 10.3945/jn.117.257386; NCT00945698; NCT00970866; NCT01239693; NCT01715038 ; PMC5697970; PUBMED: 28978680] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 2018 {published data only}
- Adams KP, Vosti SA, Ayifah E, Phiri TE, Adu‐Afarwuah S, Maleta K, et al. Willingness to pay for small‐quantity lipid‐based nutrient supplements for women and children: evidence from Ghana and Malawi. Maternal & Child Nutrition 2018;14(2):e12518. [DOI: 10.1111/mcn.12518; PUBMED: 28960913] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flax 2012 {published data only}
- Flax VL, Bentley ME, Chasela CS, Kayira D, Hudgens MG, Knight RJ, et al. Use of lipid‐based nutrient supplements by HIV‐infected Malawian women during lactation has no effect on infant growth from 0 to 24 weeks. Journal of Nutrition 2012;142(7):1350‐6. [DOI: 10.3945/jn.111.155598; NCT00164736; PMC3374670; PUBMED: 22649265] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flax 2014 {published data only}
- Flax VL, Bentley ME, Combs GF Jr, Chasela CS, Kayira D, Tegha G, et al. Plasma and breast‐milk selenium in HIV‐infected Malawian mothers are positively associated with infant selenium status but are not associated with maternal supplementation: results of the Breastfeeding, Antiretrovirals, and Nutrition study. American Journal of Clinical Nutrition 2014;99(4):950‐6. [DOI: 10.3945/ajcn.113.073833; NCT00164736; PMC3953887; PUBMED: 24500152] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haber 2016 {published data only}
- Haber JA, Solomons NW, Hampel D, Orozco MN, Allen LH. The effect of maternal supplementation with a lipid‐based nutrient supplement on infant micronutrient intake in Guatemalan women and infants. FASEB Journal 2016;30(1 Suppl):1150.3. [www.fasebj.org/doi/10.1096/fasebj.30.1_supplement.1150.3] [Google Scholar]
Hampel 2018 {published data only}
- Hampel D, Shahab‐Ferdows S, Gertz E, Flax VL, Adair LS, Bentley ME, et al. The effects of a lipid‐based nutrient supplement and antiretroviral therapy in a randomized controlled trial on iron, copper, and zinc in milk from HIV‐infected Malawian mothers and associations with maternal and infant biomarkers. Maternal & Child Nutrition 2018;14(2):e12503. [DOI: 10.1111/mcn.12503; PMC5832511; PUBMED: 28851037] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2017 {published data only}
- Johnson W, Darboe MK, Sosseh F, Nshe P, Prentice AM, Moore SE. Association of prenatal lipid‐based nutritional supplementation with fetal growth in rural Gambia. Maternal & Child Nutrition 2017;13(2):e12367. [DOI: 10.1111/mcn.12367; PMC5396370; PUBMED: 27696720] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kayira 2012 {published data only}
- Kayira D, Bentley ME, Wiener J, Mkhomawanthu C, King CC, Chitsulo P, et al. A lipid‐based nutrient supplement mitigates weight loss among HIV‐infected women in a factorial randomized trial to prevent mother‐to‐child transmission during exclusive breastfeeding. American Journal of Clinical Nutrition 2012;95(3):759‐65. [DOI: 10.3945/ajcn.111.018812; NCT00164762; PMC3278250; PUBMED: 22258269] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oaks 2017 {published data only}
- Oaks BM, Young RR, Adu‐Afarwuah S, Ashorn U, Jackson KH, Lartey A, et al. Effects of a lipid‐based nutrient supplement during pregnancy and lactation on maternal plasma fatty acid status and lipid profile: results of two randomized controlled trials. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA) 2017;117:28‐35. [DOI: 10.1016/j.plefa.2017.01.007; PMC5338685; PUBMED: 28237085] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prado 2017 {published data only}
- Prado EL, Abbeddou S, Adu‐Afarwuah S, Arimond M, Ashorn P, Ashorn U, et al. Predictors and pathways of language and motor development in four prospective cohorts of young children in Ghana, Malawi, and Burkina Faso. Journal of Child Psychology and Psychiatry 2017;58(11):1264‐75. [DOI: 10.1111/jcpp.12751; PMC5697619; PUBMED: 28543426] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schlossman 2017 {published data only}
- Schlossman N, Brown C, Batra P, Sa AB, Balan I, Balan A, et al. A randomized controlled trial of two ready‐to‐use supplementary foods demonstrates benefit of the higher dairy supplement for reduced wasting in mothers, and differential impact in infants and children associated with maternal supplement response. Food and Nutrition Bulletin 2017;38(3):275‐90. [DOI: 10.1177/0379572117700754; PUBMED: 28374648] [DOI] [PubMed] [Google Scholar]
Young 2015 {published data only}
- Young S, Natamba B, Luwedde F, Nyafwono D, Okia B, Osterbauer B, et al. "I have remained strong because of that food": acceptability and use of lipid‐based nutrient supplements among pregnant HIV‐infected Ugandan women receiving combination antiretroviral therapy. AIDS and Behavior 2015;19(8):1535‐47. [DOI: 10.1007/s10461-014-0947-0; NCT 00993031; PMC4441598; PUBMED: 25416075] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Fernald 2016 {published data only}
- Fernald LCH, Galasso E, Qamruddin J, Ranaivoson C, Ratsifandrihamanana L, Stewart CP, et al. A cluster‐randomized, controlled trial of nutritional supplementation and promotion of responsive parenting in Madagascar: the MAHAY study design and rationale. BMC Public Health 2016;16:466. [DOI: 10.1186/s12889-016-3097-7; ISRCTN14393738; PMC4891833; PUBMED: 27255923] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Adu‐Afarwuah 2011
- Adu‐Afarwuah S, Lartey A, Zeilani M, Dewey KG. Acceptability of lipid‐based nutrient supplements (LNS) among Ghanaian infants and pregnant or lactating women. Maternal & Child Nutrition 2011;7(4):344‐56. [DOI: 10.1111/j.1740-8709.2010.00286.x; PUBMED: 21496207] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arimond 2015
- Arimond M, Zeilani M, Jungjohann S, Brown KH, Ashorn P, Allen LH, et al. Considerations in developing lipid‐based nutrient supplements for prevention of undernutrition: experience from the International Lipid‐Based Nutrient Supplements (iLiNS) Project. Maternal & Child Nutrition 2015;11(Suppl 4):31‐61. [DOI: 10.1111/mcn.12049; PUBMED: 23647784] [DOI] [PMC free article] [PubMed] [Google Scholar]
Balshem 2010
- Balshem H, Helfanda M, Schünemann HJ, Oxman AD, Kunze R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI: 10.1016/j.jclinepi.2010.07.015; PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Belkacemi 2010
- Belkacemi L, Nelson DM, Desai M, Ross MG. Maternal undernutrition influences placental‐fetal development. Biology of Reproduction 2010;83(3):325‐31. [DOI: 10.1095/biolreprod.110.084517; PUBMED: 20445129] [DOI] [PubMed] [Google Scholar]
Bhatnagar 2001
- Bhatnagar S, Taneja S. Zinc and cognitive development. British Journal of Nutrition 2001;85(Suppl 2):S139‐S45. [DOI: 10.1079/BJN2000306; PUBMED: 11509102] [DOI] [PubMed] [Google Scholar]
Bhutta 2013
- Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, et al. Evidence‐based interventions for improvement of maternal and child nutrition: what can be done and at what cost?. Lancet 2013;382(9890):452‐77. [DOI: 10.1016/S0140-6736(13)60996-4; PUBMED: 23746776] [DOI] [PubMed] [Google Scholar]
Black 2013
- Black RE, Victoria CG, Walker SP, Bhutta ZA, Christian P, Onis MD, et al. Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet 2013;382(9890):427‐51. [DOI: 10.1016/S0140-6736(13)60937-X; PUBMED: 23746772] [DOI] [PubMed] [Google Scholar]
Chaparro 2010
- Chaparro CM, Dewey KG. Use of lipid‐based nutrient supplements (LNS) to improve the nutrient adequacy of general food distribution rations for vulnerable sub‐groups in emergency settings. Maternal & Child Nutrition 2010;6(Suppl 1):1‐69. [DOI: 10.1111/j.1740-8709.2009.00224.x; PUBMED: 20055936] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clapp 1998
- Clapp JF 3rd, Kiess W. Cord blood leptin reflects fetal fat mass. Journal of the Society for Gynecologic Investigation 1998;5(6):300‐3. [PUBMED: 9824809] [PubMed] [Google Scholar]
Coletta 2010
- Coletta JM, Bell SJ, Roman AS. Omega‐3 fatty acids and pregnancy. Reviews in Obstetrics and Gynecology 2010;3(4):163‐71. [PMC3046737; PUBMED: 21364848] [PMC free article] [PubMed] [Google Scholar]
Das in press
- Das JK, Salam RA, Weise Prinzo Z, Sadiq Sheikh S, Bhutta ZA. Provision of preventive lipid‐based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes. Cochrane Database of Systematic Reviews (in press). [DOI] [PMC free article] [PubMed]
De Souza 2011
- Souza AS, Fernandes FS, do Carmo MG. Effects of maternal malnutrition and postnatal nutritional rehabilitation on brain fatty acids, learning, and memory. Nutrition Reviews 2011;69(3):132‐44. [DOI: 10.1111/j.1753-4887.2011.00374.x; PUBMED: 21348877] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Dewey 2017a [pers comm]
- Dewey KG. Another iLiNS‐ZINC paper [personal communication]. Email to: ZA Bhutta 04 February 2017.
Dewey 2017b [pers comm]
- Dewey KG. RE: preliminary results of LNS reviews [personal communication]. Email to: ZA Bhutta 14 November 2016.
Diop 2003
- Diop el HI, Dossou NI, Ndour MM, Briend A, Wade SC. Comparison of the efficacy of a solid ready‐to‐use food and a liquid, milk‐based diet for the rehabilitation of severely malnourished children: a randomized trial. American Journal of Clinical Nutrition 2003;78(2):302‐7. [DOI: 10.1093/ajcn/78.2.302; PUBMED: 12885713] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI: 10.1136/bmj.315.7109.629; PMC2127453; PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
FAO/WHO/UNU 2004
- Food, Agriculture Organization of the United Nations (FAO). World Health Organization, United Nations University. Human Energy Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation. Rome, 17‐24 October 2001. Food and Nutrition Technical Report Series. Vol. 1, Rome (IT): FAO, 2004. [Google Scholar]
Forhead 2009
- Forhead AJ, Fowden AL. The hungry fetus? Role of leptin as a nutritional signal before birth. Journal of Physiology 2009;587(6):1145‐52. [DOI: 10.1113/jphysiol.2008.167072; PMC2674987; PUBMED: 19188249] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 29 April 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Haider 2015
- Haider BA, Bhutta ZA. Multiple‐micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2017, Issue 4. [DOI: 10.1002/14651858.CD004905.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI: 10.1002/sim.2380; PUBMED: 16345038] [DOI] [PubMed] [Google Scholar]
Harder 2007
- Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta‐analysis. American Journal of Epidemiology 2007;165(8):849‐57. [DOI: 10.1093/aje/kwk071; PUBMED: 17215379] [DOI] [PubMed] [Google Scholar]
Harding 2014
- Harding KL, Matias Sl, Moniruzzaman M, Stewart CP, Mridha MK, Vosti SA, et al. Rang‐Din Nutrition Study: Assessment of Participant Adherence to Lipid‐Based Nutrient and Iron‐Folic Acid Supplements among Pregnant and Lactating Women in the Context of a Study on the Effectiveness of Supplements in Bangladesh. Washington (DC): FHI 360/FANTA, 2014. [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Huybregts 2013
- Huybregts L, Roberfroid D, Lanou H, Meda N, Taes Y, Valea I, et al. Prenatal lipid‐based nutrient supplements increase cord leptin concentration in pregnant women from rural Burkina Faso. Journal of Nutrition 2013;143(5):576‐83. [DOI: 10.3945/jn.112.171181; NCT00909974; PUBMED: 23535609] [DOI] [PubMed] [Google Scholar]
Iannotti 2014
- Iannotti LL, Dulience SJL, Green J, Joseph S, François J, Anténor M‐L, et al. Linear growth increased in young children in an urban slum of Haiti: a randomized controlled trial of a lipid‐based nutrient supplement. American Journal of Clinical Nutrition 2014;99(1):198‐208. [DOI: 10.3945/%E2%80%8Bajcn.113.063883; NCT01552512; PMC3862455; PUBMED: 24225356] [DOI] [PMC free article] [PubMed] [Google Scholar]
IASC 1994
- Inter‐Agency Standing Committee. Definition of complex emergencies. interagencystandingcommittee.org/system/files/legacy_files/WG16_4.pdf (accessed prior to 16 March 2017).
iLiNS Project 2016
- UC Davis. iLiNS Project. ilins.ucdavis.edu (accessed 12 January 2016).
Imdad 2011
- Imdad A, Bhutta ZA. Effect of balanced protein energy supplementation during pregnancy on birth outcomes. BMC Public Health 2011;11(Suppl 3):S17. [DOI: 10.1186/1471-2458-11-S3-S17; PMC3231890; PUBMED: 21501434] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lanou 2014
- Lanou H, Huybregts L, Roberfroid D, Nikièma L, Kouanda S, Camp J, et al. Prenatal nutrient supplementation and postnatal growth in a developing nation: an RCT. Pediatrics 2014;133(4):e1001‐8. [DOI: 10.1542/peds.2013-2850; NCT00909974; PUBMED: 24590752] [DOI] [PubMed] [Google Scholar]
Larroque 2001
- Larroque B, Bertrais S, Czernichow P, Léger J. School difficulties in 20‐year‐olds who were born small for gestational age at term in a regional cohort study. Pediatrics 2001;108(1):111‐5. [PUBMED: 11433062] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lepercq 2001
- Lepercq J, Challier JC, Guerre‐Millo M, Cauzac M, Vidal H, Hauguel‐De Mouzon S. Prenatal leptin production: evidence that fetal adipose tissue produces leptin. Journal of Clinical Endocrinology & Metabolism 2001;86(6):2409‐13. [DOI: 10.1210/jcem.86.6.7529; PUBMED: 11397832] [DOI] [PubMed] [Google Scholar]
Lozoff 2006
- Lozoff B, Georgieff MK. Iron deficiency and brain development. Seminars in Pediatric Neurology 2006;13(3):158‐65. [DOI: 10.1016/j.spen.2006.08.004; PUBMED: 17101454] [DOI] [PubMed] [Google Scholar]
Makrides 2006
- Makrides M, Duley L, Olsen SF. Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre‐eclampsia or intrauterine growth restriction. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD003402.pub2] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, for the PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI: 10.1136/bmj.b2535] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moran 2016 [pers comm]
- Moran VH. Free access issue of MCN now available: policy, program and innovation in complementary feeding [personal communication]. Email to: ZA Bhutta 11 January 2016.
Mridha 2012
- Mridha MK, Chaparro CM, Matias SL, Hussain S, Munira S, Saha S, et al. Acceptability of Lipid‐Based Nutrient Supplements and Micronutrient Powders among Pregnant and Lactating Women and Infants and Young Children in Bangladesh and Their Perceptions about Malnutrition and Nutrient Supplements. Washington (DC): FHI 360/FANTA‐2 Bridge, 2012. [Google Scholar]
Mridha 2016a
- Mridha MK, Matias SL, Chaparro CM, Paul RR, Hussain S, Vosti SA, et al. Lipid‐based nutrient supplements for pregnant women reduce newborn stunting in a cluster‐randomized controlled effectiveness trial in Bangladesh. American Journal of Clinical Nutrition 2016;103(1):236‐49. [DOI: 10.3945/ajcn.115.111336; NCT01715038; PUBMED: 26607935] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nkhoma 2017
- Nkhoma M, Ashorn P, Ashorn U, Dewey KG, Gondwe A, Mbotwa J, et al. Providing lipid‐based nutrient supplement during pregnancy does not reduce the risk of maternal P falciparum parasitaemia and reproductive tract infections: a randomised controlled trial. BMC Pregnancy & Childbirth 2017;17:35. [DOI: 10.1186/s12884-016-1215-2; PMC5240436; PUBMED: 28095801] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nyaradi 2013
- Nyaradi A, Li J, Hickling S, Foster J, Oddy WH. The role of nutrition in children's neurocognitive development, from pregnancy through childhood. Frontiers in Human Neuroscience 2013;7:97. [DOI: 10.3389/fnhum.2013.00097; PMC3607807; PUBMED: 23532379] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ota 2015a
- Ota E, Mori R, Middleton P, Tobe‐Gai R, Mahomed K, Miyazaki C, et al. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD000230.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ota 2015b
- Ota E, Hori H, Mori R, Tobe‐Gai R, Farrar D. Antenatal dietary education and supplementation to increase energy and protein intake. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD000032.pub3] [DOI] [PubMed] [Google Scholar]
Palacios 2014
- Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem?. Journal of Steroid Biochemistry and Molecular Biology 2014;144(Pt A):138‐45. [DOI: 10.1016/j.jsbmb.2013.11.003; NIHMS541186; PMC4018438; PUBMED: 24239505] [DOI] [PMC free article] [PubMed] [Google Scholar]
Phuka 2008
- Phuka JC, Maleta K, Thakwalakwa C, Cheung YB, Briend A, Manary MJ, et al. Complementary feeding with fortified spread and incidence of severe stunting in 6‐ to 18‐month‐old rural Malawians. Archives of Pediatrics & Adolescent Medicine 2008;162(7):619‐26. [DOI: 10.1001/archpedi.162.7.619; NCT00131209; PMC3721756; PUBMED: 18606932] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Risnes 2011
- Risnes KR, Vatten LJ, Baker JL, Jameson K, Sovio U, Kajantie E, et al. Birthweight and mortality in adulthood: a systematic review and meta‐analysis. International Journal of Epidemiology 2011;40(3):647‐61. [DOI: 10.1093/ije/dyq267; PUBMED: 21324938] [DOI] [PubMed] [Google Scholar]
Schofield 2009
- Schofield D, Huffman SL, Young Child Nutrition Working Group: Formulation Subgroup. Formulations for fortified complementary foods and supplements: review of successful products for improving the nutritional status of infants and young children. Food and Nutrition Bulletin 2009;30(2):S239‐55. [PUBMED: 20496618] [PubMed] [Google Scholar]
Shenkin 2004
- Shenkin SD, Starr JM, Deary IJ. Birthweight and cognitive ability in childhood: a systematic review. Psychological Bulletin 2004;130(6):989‐1013. [DOI: 10.1037/0033-2909.130.6.989; PUBMED: 15535745] [DOI] [PubMed] [Google Scholar]
Stewart 2017 [pers comm]
- Stewart CP. RE: [personal communication]. Email to: ZA Bhutta 27 April 2017.
Thakwalakwa 2010
- Thakwalakwa C, Ashorn P, Phuka J, Cheung YB, Briend A, Puumalainen T, et al. A lipid‐based nutrient supplement but not corn‐soy blend modestly increases weight gain among 6‐ to 18‐month‐old moderately underweight children in rural Malawi. Journal of Nutrition 2010;140(11):2008‐13. [DOI: 10.3945/jn.110.122499; NCT00420368; PUBMED: 20861218] [DOI] [PubMed] [Google Scholar]
UNICEF 2014
- UNICEF. Humanitarian action for children 2014: overview. www.unicef.org/gambia/Humanitarian_Action_for_Childen_2014_Overview.pdf (accessed 22 November 2015).
Vosti 2015
- Vosti S, Humber J, Phiri T, Ashorn P, Maleta K, Dewey K. The cost of home delivery schemes for lipid‐based nutrient supplement products: a policy experiment from rural Malawi. European Journal of Nutrition & Food Safety 2015;5(5):1053‐4. [DOI: 10.9734/EJNFS/2015/21238] [DOI] [Google Scholar]
WHO 2007
- World Health Organization (WHO), World Food Programme (WFP), United Nations System Standing Committe on Nutrition (UNSSCN), United Nations Children's Fund (UNICEF). Community‐Based Management of Severe Acute Malnutrition: A Joint Statement by the World Health Organization, the World Food Programme, the United Nations System Standing Committee on Nutrition and the United Nations Children's Fund. Geneva: WHO; WFP; UNSSCN; UNICEF, 2007. [Google Scholar]
WHO 2012
- World Health Organization (WHO). Supplementary Foods for the Management of Moderate Acute Malnutrition in Infants and Children 6‐59 Months of Age: Technical Note. Geneva: WHO, 2012. [Google Scholar]
WHO 2013
- World Health Organization (WHO). Guideline: Updates on the Management of Severe Acute Malnutrition in Infants and Children. Geneva: WHO, 2013. [PubMed] [Google Scholar]
WHO 2014
- World Health Organization (WHO). Global Nutrition Targets 2025: Low Birth Weight Policy Brief. Geneva: WHO, 2014. [Google Scholar]
WHO 2015
- World Health Organization (WHO). The Global Prevalence of Anaemia in 2011. Geneva: WHO, 2015. [Google Scholar]
WHO 2016
- World Health Organization (WHO). Use of Multiple Micronutrient Powders for Point‐Of‐Use Fortification of Foods Consumed by Pregnant Women. Geneva: WHO, 2016. [PubMed] [Google Scholar]
Zeisel 2006
- Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annual Review of Nutrition 2006;26:229‐50. [DOI: 10.1146/annurev.nutr.26.061505.111156; NIHMS50836; PMC2441939; PUBMED: 16848706] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zimmermann 2011
- Zimmermann MB. The role of iodine in human growth and development. Seminars in Cell & Developmental Biology 2011;22(6):645‐52. [DOI: 10.1016/j.semcdb.2011.07.009; PUBMED: 21802524] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Das 2017
- Das JK, Salam RA, Weise Prinzo Z, Hoodbhoy Z, Bhutta ZA. Lipid‐based nutrient supplements for pregnant women and their impact on pregnancy, birth, and infant developmental outcomes in stable and emergency settings. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD012610] [DOI] [PMC free article] [PubMed] [Google Scholar]


