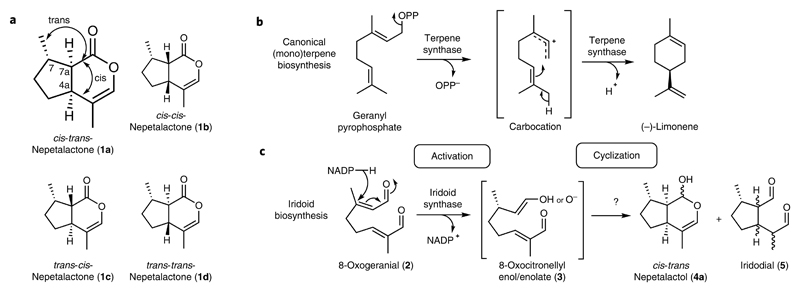
Fig. 1. Nepetalactones and terpenoid biosynthesis.
a, Nepetalactone 1 stereoisomers observed in Nepeta species. b, Representative canonical terpene biosynthesis mechanism (limonene synthase). Typical terpene synthases activate linear precursors by removal of diphosphate or protonation. The activated carbocation intermediates undergo selective cyclization inside the same terpene synthase active site. c, Iridoid biosynthesis mechanism. Iridoid synthase activates its linear precursor (8-oxogeranial (2)) by reduction to form the 8-oxocitronellyl enol/enolate intermediate 3, which cyclizes to form a mixture of cis-trans-nepetalactol (4a) and iridodials (5).