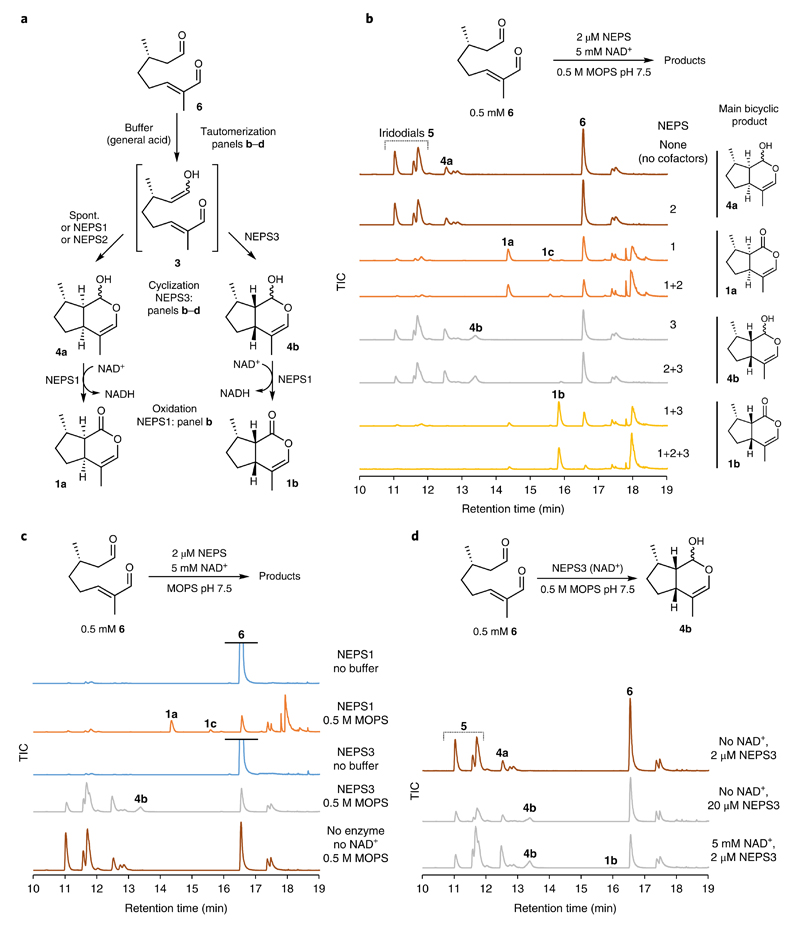
Fig. 4. NEPS activities explored with (S)-8-oxocitronellal (6).
a, Summary of NEPS enzyme activities described in this figure. b, NEPS activities with 6, buffer and NAD+, presented as GC–MS TICs. The panel largely recapitulates observations of Fig. 3b, but in the absence of ISY. Unknown side products are formed by NEPS1. c, Buffer dependence of NEPS activity with 6. In the absence of buffer, NEPS1 and NEPS3 have no detectable activity; addition of buffer reveals enzyme activities. Buffer-catalyzed tautomerization of 6 appears to be necessary for enzyme activity, supporting the hypothesis that the activated 3, and not 6, is the key NEPS substrate. d, NEPS3-catalyzed cyclization. The addition of NAD+ is not required for NEPS3 cyclization activity, though addition does promote the reaction. We hypothesize that the cyclization is not oxidoreductive, but NAD+ acts in a nonchemical manner (that is, protein stabilization). All reactions were incubated for 16 h (in contrast to 3h for Fig. 3) and are presented as GC–MS TICs. All experiments were performed independently twice with similar results.