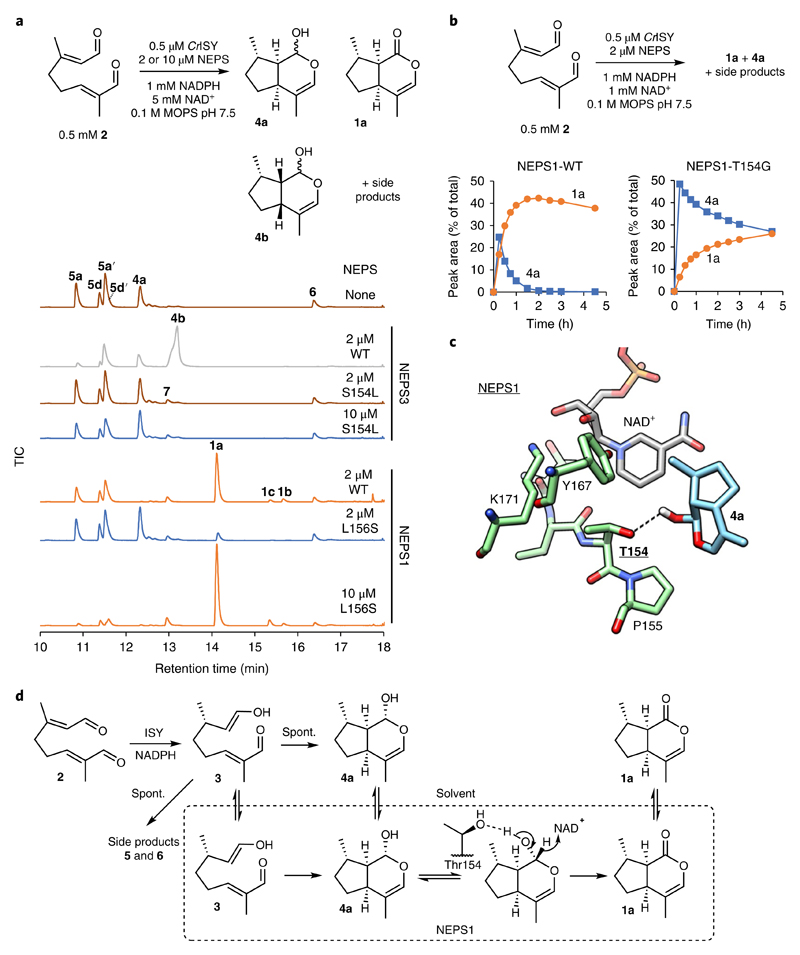
Fig. 6. NEPS variants.
a, Coupled assay with 8-oxogeranial (2), ISY and NEPS variants. NEPS3-S154L has no cis-cis cyclase activity (no 4b), but at 10 μM enzyme the formation of 4a is increased. NEPS1-L156S has decreased dehydrogenase activity compared NEPS1 (less 1a) but no cis-cis cyclase activity (no 4b). Experiments were performed independently twice with similar results. b, Time course with 2, ISY and NEPS1-WT or NEPS1-T154G. Quantities of 4a (blue) and 1a (orange) are reported as percent proportion of total product peak area. NEPS1-T154G has lower dehydrogenation activity compared to WT (conversion of 4a into 1a). See Supplementary Fig. 17 for all TICs of the time course and Supplementary Table 2 for kinetic analysis. Full time-course experiments were performed once, but conversions after 3 h were performed independently twice with similar results. c, Putative binding mode of 4a in the NEPS1 homology model active site. The putative hydrogen bond interaction between the lactol and Thr154 is highlighted with a dotted line. The depicted binding mode was ranked third of ten binding modes (rank 1 = -6.4 kcal.mol−1, depicted rank 3 = -6.3 kcal.mol−1). d, Scheme of NEPS1 activities and interactions. NEPS1 has distinct cyclization and dehydrogenation activities. The behavior of NEPS1-T154G suggests that Thr154 is involved in dehydrogenation. The two activities may involve different active site interactions. The slightly improved cyclase activity of T154G may be due to poor binding of 4a, which frees more enzyme for binding and cyclizing 3 into 4a.