Abstract
Chronic pancreatitis (CP) is a fibrotic disorder of the pancreas leading to clinical sequelae like pain and an excess of comorbidity including cardiovascular disease and cancers. The aim of this study was to determine the relationship between systemic inflammation and quality of life in patients with CP. Patients were prospectively recruited and underwent a quality of life assessment (EORTC QLQ-C30 and PAN 28). The serum inflammatory profile was assessed using an MSD 30-plex array. The relationship between clinical variables, inflammatory cytokines and quality of life was determined by a GLM-MANOVA and the individual impact of significant variables evaluated by a second ANOVA. In total, 211 patients with a median age of 53 years were recruited across 5 European centres. Gender, age, nicotine and alcohol abuse were clinical variables associated with altered quality of life. Systemic inflammation with high levels of pro-inflammatory cytokines (Eotaxin, IL-1β, IL-7, IL-8, IL-12/IL-23p40, IL-12p70, IL-13, IL-16, IP-10, MCP-1, MCP-4, MDC, MIP-1a, TARC, TNFß) was associated with diminished quality of life in general and specific domains including pain, physical and cognitive functioning. As conclusion, CP is associated with a systemic inflammatory response that has a negative impact on quality of life and accelerates aging.
Subject terms: Chronic pancreatitis, Pancreas
Introduction
Chronic pancreatitis (CP) is a disease characterised by chronic inflammation in the pancreas which ultimately results in organ fibrosis. This process manifests itself clinically in a variety of ways including chronic abdominal pain, steatorrhoea, diabetes mellitus, obstructive jaundice and malabsorption of nutrients leading to secondary conditions such as osteoporosis1–3. Unsurprisingly CP is associated with diminished quality of life and a high socioeconomic burden not only to individuals but also to society as a whole3–5.
Medical co-morbidity is commonplace in patients with CP. In a retrospective registry study in Denmark Bang et al. compared the outcome of 11,972 CP patients with 119,720 age and gender matched controls over a 15-year period6. They demonstrated that there was a marked excess mortality in patients with CP over the study period with a mean age of death in patients of 63.7 years as compared to 72.1 years in controls (p < 0.0001). There was an excess of comorbidity in the CP group (78% vs. 38%; p < 0.0001) with diseases including cerebrovascular disease, COPD, ulcer disease, diabetes mellitus and chronic renal disease. Further there was an increased risk of malignancy in the CP group (13.6% vs. 7.9%; p < 0.0001)6.
These findings fit with the concept that CP is associated with accelerated biological ageing a process typically characterized by the development of frailty, sarcopenia and diseases associated with older age such as cardiovascular disease and cancers7. Specifically there is a recognized increase in the incidence of pancreatic ductal adenocarcinoma in patients with CP, especially in the hereditary form of the disease, which is attributed predominantly to the local inflammatory process acting as an oncogenic driver8,9.
While shared risk factors and co-morbidities like smoking and malnutrition contribute to accelerated aging in patients with CP, there is also a strong and established association between accelerated ageing and systemic inflammation – the so-called inflammageing phenotype. Indeed an elevation in markers of systemic inflammation e.g. serum IL-6 and CRP occurs during the normal ageing process despite the absence of infection or other pathophysiological processes to account for their rise10,11. In a recent systematic review we have demonstrated that CP is associated with elevated systemic levels of inflammatory mediators such as IL-6, TNFα, IL-8 and members of the IL-1 family12. The aim of the current study was to determine the impact of this systemic inflammation on quality of life in patients with CP and thereby determine if inflammageing may be a concept worthy of further exploration in this disease.
Materials and Methods
The study was approved by the ethical review board of Technische Universität München (Project 104/15) on 02/04/2015 and by each of the relevant ethics authorities in the participating centres. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. Written, informed consent was obtained from each patient included in the study.
Patients
Patients presenting with an established diagnosis of CP attending for review at each of the participating centres were invited to participate in this study. The presence of CP was defined as per the HaPanEU guidelines i.e. either a pain history typical for the disease or, in the absence of pain, the presence of biochemically proven pancreatic exocrine insufficiency. In addition all patients were required to have radiological evidence of CP and the initial diagnosis had to be at least 2 years prior to recruitment2.
The exclusion criteria for this study were as follows: an active diagnosis of neoplasia or a disease free interval of less than five years; patients unable to provide informed consent to participate in the study including those less than 18 years of age; patients with Childs-Pugh score B/C liver cirrhosis; patients who were immunosuppressed either as a result of disease (e.g. HIV) or active medical treatment (patients with NSAID as pain medication were not excluded); patients treated with antibiotic therapy within the last month or with an active infective process; patients who had received systemic steroid treatment within the last month; patients with chronic kidney disease stage 4 or greater (eGFR < 30 ml/min) and those who had received surgical treatment of any form to any body-site within the last year.
At the point of recruitment the interviewing doctor completed a study questionnaire and all patients were asked to complete the EORTC QLQC-30 and PAN-28 questionnaires. Blood samples were taken for full blood count, urea & electrolytes, liver function, CRP and HbA1C. A serum sample was stored for later analysis at −80 °C.
Interpretation of quality of life data
Quality of life was assessed by calculating function or symptom scores for each of the domains assessed by the two questionnaires4,13. For both types of score a raw score was first calculated as the mean response to each of the component items constituting the domain. For functional scores the following equation was then utilised:
For functional domains a low score implies poorer function and therefore a lower quality of life. In contrast for symptom domains the following formula was utilised:
For symptom domains a high score implies more severe symptoms and therefore a poorer quality of life.
Assessment of inflammatory response
Using stored serum the concentration of 28 inflammatory mediators (Eotaxin, Eotaxin-3, GM-CSF, IFNγ, IL-10, IL-12/IL-23p40, IL-12p70, IL-13, IL-15, IL-16, IL-17, IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-7, IL-8, IP-10, MCP-1, MCP-4, MDC, MIP-1α, MIP-1β, TARC, TNFα, TNF-β, VEGF-A) were assessed using a multiplex array according to the manufacturers instructions (Meso Scale Diagnostics, Maryland, USA).
To determine the relationship between systemic inflammation and quality of life patients with CP were compared to each other and not to healthy controls. Therefore each of the inflammatory mediators measured was categorised as either ‘high’ or ‘low’ based upon the median serum level.
Statistical analysis
To determine the relationship between the measured variables and quality of life measures we performed a general linear model multivariate analysis of variance (GLM-MANOVA) separately for the QLQ-C30 and PAN-28 questionnaires. We assessed the impact of each factor using Wilks λ with p < 0.05 being considered significant. To ensure that the associations of measured inflammatory variables were independent of key clinical factors (smoking, diabetes mellitus and current alcohol consumption) the analysis was repeated with these factors as co-variates14.
Those factors identified as being associated with quality of life on GLM-MANOVA were then entered into a second ANOVA to investigate the association between each factor and the quality of life scale items individually with post-hoc testing using the Bonferroni correction with a p < 0.05 being considered statistically significant.
Numerical variables are presented as mean (±standard deviation). All statistical analysis was carried out using SPSS v. 23 (IBM Corporation, USA).
Any additional data that is not mentioned in the article is provided as supplementary material.
Results
Across the five participating centres 296 patient were screened for this study, 51 met one or more of the exclusion criteria, 30 refused to participate, 3 were discharged before inclusion and 1 patient had an unsure diagnosis of CP. Altogether a total of 211 patients were recruited for this study. Patient characteristics are summarised in Table 1.
Table 1.
Summary of patient characteristics.
| Participant Age | 53 (19–84) years# |
|---|---|
| Gender | 156 Male/55 Female |
| Time since initial diagnosis of CP | 7 (2–53) years # |
| Pancreatitis Aetiology Alcohol Other |
148 (70.1%) 63 (29.9%) |
| Current Smoker (n = 210)* | 108 (51.4%) |
| Current Drinker (n = 209)* | 55 (26.3%) |
| Diabetes Mellitus (n = 210)* | 89 (42.4%) |
| Pain medication with NSAID Mild opioids (WHO class 2) Potent opioids (WHO class 3) |
77 (36.5%) 59 (28.0%) 51 (24.2%) |
| Pancreatic surgery | 40 (19.0%) |
*Numbers in parenthesis represent number of patients with complete data for the variable; #Median (range); WHO = world health organisation.
Quality of life measures
Across the entire study population the mean global quality of life score was 51.54(±25.38). In the QLQ-C30 questionnaire the lowest function scores were reported for role functioning (60.43 ± 35.60), emotional functioning (61.40 ± 35.60) and social functioning (62.51 ± 34.23) and the highest symptom scores were reported for pain (50.65 ± 34.86), insomnia (48.22 ± 37.61) and fatigue (47.91 ± 30.72). For the PAN-28 questionnaires the highest symptom scores were reported for fear for future health (65.34 ± 33.40), bloated abdomen (47.45 ± 37.79) and pancreatic pain (46.90 ± 31.20). Table 2 summarises quality of life scores across each domain.
Table 2.
Calculated scores of EORTC QLQ-C30 and PAN28 scales.
| Scales | Items | Mean (SD) |
|---|---|---|
| QLQ-C30 | ||
| Global quality of life | Q29,30 | 51.54 (25.38) |
| Physical functioning | Q1-5 | 73.33 (24.97) |
| Role functioning | Q6-7 | 60.43 (35.60) |
| Emotional functioning | Q21-24 | 61.40 (29.35) |
| Cognitive functioning | Q20,25 | 72.79 (26.66) |
| Social functioning | Q26,27 | 62.51 (34.23) |
| Fatigue | Q10,12,18 | 47.91 (30.72) |
| Nausea/Vomiting | Q14-15 | 25.09 (31.57) |
| Pain | Q9,19 | 50.65 (34.86) |
| Dyspnea | Q8 | 24.43 (31.44) |
| Insomnia | Q11 | 48.22 (37.61) |
| Appetite loss | Q13 | 38.37 (37.08) |
| Constipation | Q16 | 20.65 (29.92) |
| Diarrhea | Q17 | 22.77 (28.96) |
| Financial problems | Q28 | 35.50 (36.47) |
| PAN-28 | ||
| Pancreatic pain | Q31,33,35 | 46.90 (31.20) |
| Digestive function | Q36,37 | 45.40 (36.61) |
| Jaundice | Q44,45 | 14.17 (20.03) |
| Altered bowel functioning | Q46,47 | 30.47 (28.05) |
| Body image | Q48,51 | 37.89 (32.48) |
| Alcohol related guilt | Q49,50 | 21.15 (28.40) |
| Satisfaction with health care | Q55,56 | 38.85 (35.71) |
| Sexual functioning | Q57,58 | 38.83 (38.43) |
| Bloated abdomen | Q32 | 47.45 (37.79) |
| Night pain | Q34 | 37.75 (36.06) |
| Taste changes | Q38 | 20.03 (29.99) |
| Indigestion | Q39 | 36.32 (36.09) |
| Flatulence | Q40 | 42.79 (33.74) |
| Weight loss | Q41 | 30.36 (38.67) |
| Decreased muscle strength | Q42 | 36.50 (34.83) |
| Dry mouth | Q43 | 38.26 (34.76) |
| Fear for future health | Q53 | 65.34 (33.40) |
| Ability to plan ahead | Q54 | 41.62 (37.39) |
Q = question.
Clinical variables associated with quality of life
Using the GLM-MANOVA we identified that age (p = 0.03), gender (p = 0.0001) and current smoking status (p = 0.002) were all associated with quality of life outcomes in the QLQ-C30 questionnaire (Supplementary Table 1). In the PAN-28 questionnaire gender (p = 0.04), being a current drinker (p = 0.001) and the presence of diabetes mellitus (p = 0.04) were also negatively associated with quality of life outcomes (Supplementary Table 2).
Subsequent ANOVA analysis revealed that being a current smoker impacted negatively on quality of life across a total of 14 domains in the QLQ-C30 quality of life questionnaire. Male gender was associated with poorer cognitive function, increased dyspnoea scores but better digestive function. Older age was associated with increased financial difficulties. In the PAN 28 being a current drinker was associated with a higher feeling of alcohol related guilt but was associated with less night pain, lower symptom scores for decreased muscle strength and a positive impact on body image. The presence of diabetes mellitus was associated with poorer sexual function but less alcohol related guilt (Table 3).
Table 3.
Impact of patient characteristics on quality of life.
| Predictive Variable | Questionnaire | Quality of Life Domain(s) | Mean Score (Male) | Mean Score (Female) | Bonferroni p-value |
|---|---|---|---|---|---|
| Gender | QLQ-C30 | Cognitive Function | 70.1 | 80.9 | 0.01 |
| Dyspnoea | 27.4 | 15.2 | 0.02 | ||
| PAN-28 | Digestive Function | 60.7 | 42.3 | 0.02 | |
| Mean Score (Yes) | Mean Score (No) | ||||
| Older age | QLQ-C30 | Financial Difficulties | 43.7 | 27.6 | 0.002 |
| Current Drinker | PAN-28 | Body Image | 27.8 | 40.7 | 0.02 |
| Alcohol Related Guilt | 28.5 | 15.3 | 0.007 | ||
| Night Pain | 29.9 | 42.7 | 0.05 | ||
| Decreased Muscle Strength | 28.5 | 41.3 | 0.04 | ||
| Smoker | QLQ-C30 | Global Quality of Life | 47.8 | 56.1 | 0.02 |
| Physical Functioning | 67.1 | 80.3 | <0.0001 | ||
| Role Functioning | 50.9 | 70.4 | <0.0001 | ||
| Emotional Functioning | 55.7 | 68.0 | 0.004 | ||
| Cognitive Function | 66.5 | 80.0 | <0.0001 | ||
| Social Function | 53.4 | 72.6 | <0.0001 | ||
| Fatigue | 55.5 | 40.2 | <0.0001 | ||
| Nausea and Vomiting | 33.2 | 16.5 | <0.0001 | ||
| Pain | 59.8 | 42.3 | <0.0001 | ||
| Dyspnoea | 30.2 | 18.2 | 0.007 | ||
| Insomnia | 59.1 | 37.9 | <0.0001 | ||
| Appetite Loss | 48.8 | 27.0 | <0.0001 | ||
| Constipation | 28.9 | 12.6 | <0.0001 | ||
| Financial Difficulties | 43.0 | 29.8 | 0.01 | ||
| Diabetes mellitus | Sexual Function | 31.9 | 48.1 | 0.01 | |
| Alcohol Related Guilt | 11.8 | 25.5 | 0.003 |
Systemic inflammation and quality of life
Mean levels of inflammatory mediators are presented in Supplementary Table 3. The most relevant associations of inflammatory mediators with quality of life are presented in Table 4 and Fig. 1. Supplementary Table 4 displays all associations with quality of life domains and inflammatory mediators.
Table 4.
Inflammatory mediators and quality of life.
| Predictive Variable | Questionnaire | Quality of Life Domain(s) | Mean Score (Low) | Mean Score (High) | Bonferroni p-value |
|---|---|---|---|---|---|
| Eotaxin | PAN-28 | Bowel Function | 24.5 | 34.9 | 0.03 |
| Abdominal Bloating | 54.6 | 37.6 | 0.009 | ||
| IL-7 | QLQ-C30 | Global Quality of Life | 55.8 | 48.2 | 0.04 |
| Fatigue | 43.5 | 53.1 | 0.03 | ||
| Nausea and Vomiting | 20.0 | 29.8 | 0.03 | ||
| Dyspnoea | 19.3 | 30.4 | 0.01 | ||
| Diarrhoea | 17.2 | 27.4 | 0.01 | ||
| IL-8 | QLQ-C30 | Global Quality of Life | 56.0 | 48.3 | 0.04 |
| Physical Functioning | 78.9 | 67.8 | 0.002 | ||
| Cognitive Functioning | 77.5 | 68.3 | 0.02 | ||
| Nausea and Vomiting | 20.0 | 29.4 | 0.04 | ||
| Dyspnoea | 20.1 | 29.1 | 0.05 | ||
| Appetite Loss | 32.6 | 44.0 | 0.03 | ||
| IL-12/IL-23p40 | QLQ-C30 | Dyspnoea | 29.6 | 29.5 | 0.03 |
| IL-16 | QLQ-C30 | Global Quality of Life | 56.7 | 47.5 | 0.01 |
| Physical Functioning | 78.4 | 68.2 | 0.005 | ||
| Cognitive Functioning | 77.5 | 68.1 | 0.02 | ||
| Fatigue | 43.0 | 53.3 | 0.02 | ||
| Nausea and Vomiting | 17.2 | 32.3 | 0.001 | ||
| Dyspnoea | 19.2 | 30.1 | 0.02 | ||
| IP-10 | QLQ-C30 | Dyspnoea | 20.2 | 29.3 | 0.04 |
| PAN-28 | Bowel Function | 19.7 | 38.3 | <0.0001 | |
| MCP-4 | PAN-28 | Bowel Function | 23.8 | 34.8 | 0.02 |
| MDC | QLQ-C30 | Physical Functioning | 78.7 | 67.8 | 0.003 |
| Fatigue | 43.1 | 53.3 | 0.02 | ||
| Nausea and Vomiting | 17.2 | 32.4 | 0.001 | ||
| Dyspnoea | 17.9 | 31.5 | 0.002 | ||
| Bowel Function | 22.0 | 36.1 | 0.002 | ||
| Weight Loss | 24.2 | 39.9 | 0.02 | ||
| MIP-1a | QLQ-C30 | Global Quality of Life | 56.5 | 47.6 | 0.02 |
| Physical Functioning | 80.2 | 66.2 | <0.0001 | ||
| Cognitive Functioning | 78.3 | 67.2 | 0.004 | ||
| Fatigue | 40.9 | 55.6 | 0.001 | ||
| Nausea and Vomiting | 17.6 | 32.1 | 0.001 | ||
| Dyspnoea | 18.6 | 30.8 | 0.007 |
Figure 1.
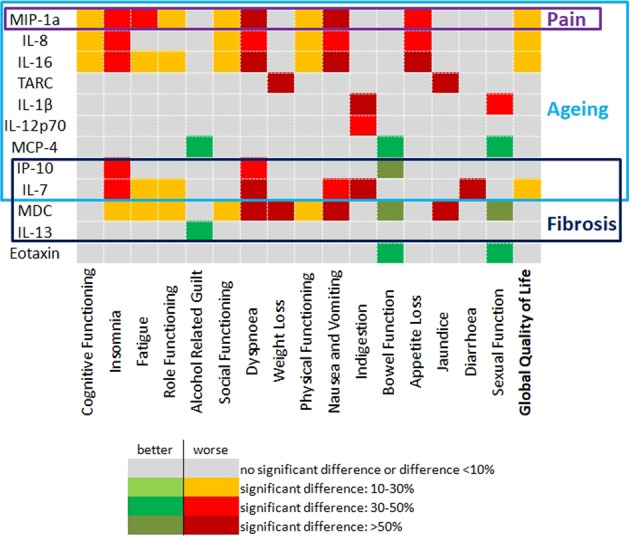
Important aspects of systemic inflammation on quality of life.
Of the measured inflammatory mediators 3 were associated with an impact on quality of life across both QLQ-C30 and PAN-28, these being MDC, IL-7 and IP-10. Across the two questionnaires a high serum MDC level was associated with a negative impact across 12 measured quality of life domains including role functioning, social functioning, fatigue and insomnia. A high serum IL-7 had a negative impact on a total of 10 quality of life domains across the two questionnaires including global quality of life. High IL-7 seemed to be particularly associated with a negative impact on domains related to digestive function including nausea and vomiting, diarrhoea and indigestion. A high serum IP-10 had a negative impact on quality of life across a total of 4 domains.
High expression of a further 5 inflammatory mediators (IL-8, IL-12/IL-23p40, IL-16, MIP1α and TNFβ) were associated with a negative impact on quality of life domains measured by QLQ-C30. Of these high serum levels of IL-8, IL-16 and MIP1α were associated with a negative impact on global quality of life. Individually a high serum MIP1α had a negative impact across 12 measured domains, IL-16 across 10 domains, IL-8 across 8 domains and both IL-12/IL-23p40 and TNFβ on one domain each.
For the PAN-28 questionnaire a high expression of 6 of the measured inflammatory mediators (Eotaxin, IL-12p70, IL-13, IL-1β, MCP1, MCP4, TARC) was associated with a negative impact on quality of life. Whilst a high serum MIP1β was associated with a negative impact on quality of life this association was lost when the GM-MANOVA analysis was adjusted for diabetes mellitus and it was thus not considered further (λ = 0.802; p = 0.07, Supplementary Table 2). A high serum MCP4 had a negative impact on 4 of the measured quality of life domains. Eotaxin, MCP-1 and TARC impacted on 3 domains each; IL-12p70 and IL-1β on 2 domains each and IL-13 on one domain.
Discussion
It has been reported in a variety of other diseases that elevated serum inflammatory cytokine levels are associated with an impaired quality of life in patients15–17. In this study we have shown that there is a strong link between a diminished quality of life and systemic inflammation in patients with CP. Several pro-inflammatory mediators are associated with reduced quality of life in general or with worse specific function and symptom scores of the QLQ-C30 and PAN 28 questionnaires. While some of the inflammatory mediators were described in recent clinical and pre-clinical studies dealing with this issue, changed serum levels of most of the identified inflammatory mediators have not been associated to CP yet12,18. Patients with CP suffer from a diminished quality of life in general. Our results indicate three aspects with a particular impact on quality of life: pain as the most prevalent symptom, pro-inflammatory mediators involved in fibrosis as well as in accelerated aging.
Amongst the symptom scores pain stands out with an apparently high score. This finding is in accordance with previous studies and questions the current analgesic treatment of these patients. Analgesic medication alone often does not result in sufficient pain control13,19. Otherwise, it has been demonstrated that smoking influences disease progression in chronic pancreatitis as measured by calcifications within the gland and the development of diabetes mellitus20. Of the measured clinical variables smoking impacted on 14 individual domains of the QLQ-C30 questionnaire including those such as pain and global quality of life. This confirms the results of recent publications that also showed that pain and nicotine abuse negatively influence quality of life in patients with CP21,22. Whether pain and smoking status are independent variables influencing quality of life as described by Machicado et al. or nicotine abuse influences quality of life by its impact on pain still has to be evaluated. The fact that smoking cessation might be a potent additive option to treat pain and improve quality of life in those patients highlights the need for targeted support for this patient cohort.
In this study we have shown for the first time, to our knowledge, an association between an elevated MIP-1a level and poorer quality of life. MIP-1a is primarily described as a chemokine attracting macrophages and neutrophils to areas of tissue injury23, indeed MIP-1a is thought to play a key role in attracting macrophages to the injured pancreas in murine models of alcohol induced pancreatitis24.
Whilst it may be that MIP-1a is simply a surrogate for pancreatic injury and therefore a diminished quality of life the chemokine has been linked with more systemic effects that may also affect quality of life. For example elevated serum levels of MIP-1a have been linked to changes in mood and altered cognition and this would fit with our finding that MIP-1a was significantly associated with poorer cognitive functioning25,26. There is also evidence from animal models of sciatic nerve ligation that MIP-1a contributes to the pathogenesis of neuropathic pain although we did not demonstrate an association with pain in the current study27.
Fibrosis is one key element in pathogenesis and progression of CP and driven among others by Il-1328. Also, an increased serum MDC level was associated with a negative impact on quality of life across both questionnaires. MDC is associated with pulmonary fibrosis and in those patients with idiopathic pulmonary fibrosis, it is associated with poorer outcomes29,30. Similarly whilst little is known of the role of IL-7 in CP it does play a fundamental role in the biology of fibroblast activation. Also IP-10 has been identified as a biomarker of chronic liver disease where it promotes inflammation and fibrosis and so may represent a part of the fibrogenic process in CP31,32.
Another area that has not received much exploration to date is the impact of systemic inflammation on the development of frailty and ageing. Typically ageing is associated with triggering of the NF-κB, IL-1α, TGF-β and IL-6 pathways33. In addition, a senescence associated secretory phenotype has been described and many inflammatory mediators identified in this analysis belong to this secretory phenotype. As a negative regulator of TGF-β signalling, Il-7 is a component of the senescence associated phenotype34,35. An elevated serum IP-10 level is also considered to be a biomarker of the ageing process36. Of the other inflammatory mediators identified as having an association with quality of life in this study several are known to be associated with senescence and ageing including IL-8, MIP-1a, IL-13, IL-1β and MCP-435. Declines in both physical and cognitive functioning are key hallmarks of the ageing process. Il-8 and 16 as well as MDC and MIP-1a are associated with significantly lower scores for physical and cognitive functioning.
In addition several of the other inflammatory mediators are associated with poorer quality of life and ageing in other diseases. For example an elevated serum IL-16 level is associated with neurocognitive impairment in those living with HIV and an elevated serum IL-12p70 is associated with poorer cognitive function in ageing adults37,38.
Whether cellular senescence is the cause of the inflammatory pattern seen in those patients with a poorer quality of life, or, whether it is merely a consequence of ongoing cellular injury within the pancreas remains to be determined although there is evidence that senescence does occur within pancreatic acinar cells and stellate cells in patients with CP39,40.
Further studies are needed to evaluate whether the inflammatory mediators are causative for the recorded symptoms and changes in quality of life and whether these inflammatory mediators might then qualify as potential therapeutic targets to treat symptoms and increase quality of life in patients with CP.
Conclusion
This study has demonstrated that diminished quality of life in CP is associated with elevated serum levels of several inflammatory mediators which cannot be explained by clinical variables such as smoking status and diabetes mellitus. Also inflammatory mediators might be involved in disease progression and accelerated aging in patients with CP. It remains to be determined whether this effect is causal or merely a surrogate for ongoing pancreatic injury but it is worthy of further exploration given the strong association with CP and poor systemic health. In addition, smoking has a strong influence on pain and quality of life in general in those patients.
Supplementary information
Acknowledgements
This work was carried out as part of a collaboration formed through the Pancreas 2000 programme. This research was funded by the National Institute for Health Research Newcastle Biomedical Research Centre based at Newcastle Hospitals NHS Foundation Trust and Newcastle University. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Author Contributions
S.M.R., S.R., S.B., I.V., A.M., P.M., R.M.C. and J.R. contributed to the design of the study. S.M.R., S.R., S.B., I.V., A.M. and E.S. were responsible for data collection. J.M. provided scientific expertise for laboratory analysis. S.M.R., S.R. and P.M. analysed the data. S.M.R. and S.R. drafted the manuscript and all authors edited this.
Data Availability
All data generated or analysed during this study, that are not included in this published article (and its Supplementary Information Files), are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Stuart M. Robinson and Sebastian Rasch contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43846-8.
References
- 1.Martinez-Moneo E, et al. Deficiency of fat-soluble vitamins in chronic pancreatitis: A systematic review and meta-analysis. Pancreatology: official journal of the International Association of Pancreatology. 2016;16:988–994. doi: 10.1016/j.pan.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Lohr JM, et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU) United European gastroenterology journal. 2017;5:153–199. doi: 10.1177/2050640616684695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jupp J, Fine D, Johnson CD. The epidemiology and socioeconomic impact of chronic pancreatitis. Best practice & research. Clinical gastroenterology. 2010;24:219–231. doi: 10.1016/j.bpg.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Fitzsimmons D, et al. Symptoms and quality of life in chronic pancreatitis assessed by structured interview and the EORTC QLQ-C30 and QLQ-PAN26. The American journal of gastroenterology. 2005;100:918–926. doi: 10.1111/j.1572-0241.2005.40859.x. [DOI] [PubMed] [Google Scholar]
- 5.Wehler M, et al. Health-related quality of life in chronic pancreatitis: a psychometric assessment. Scandinavian journal of gastroenterology. 2003;38:1083–1089. doi: 10.1080/00365520310005956. [DOI] [PubMed] [Google Scholar]
- 6.Bang UC, Benfield T, Hyldstrup L, Bendtsen F, Beck Jensen JE. Mortality, cancer, and comorbidities associated with chronic pancreatitis: a Danish nationwide matched-cohort study. Gastroenterology. 2014;146:989–994. doi: 10.1053/j.gastro.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 7.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Kirkegard J, Mortensen FV, Cronin-Fenton D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. The American journal of gastroenterology. 2017;112:1366–1372. doi: 10.1038/ajg.2017.218. [DOI] [PubMed] [Google Scholar]
- 9.Garcea G, Dennison AR, Steward WP, Berry DP. Role of inflammation in pancreatic carcinogenesis and the implications for future therapy. Pancreatology: official journal of the International Association of Pancreatology. 2005;5:514–529. doi: 10.1159/000087493. [DOI] [PubMed] [Google Scholar]
- 10.Fougere, B., Boulanger, E., Nourhashemi, F., Guyonnet, S. & Cesari, M. Chronic Inflammation: Accelerator of Biological Aging. The journals of gerontology. Series A, Biological sciences and medical sciences, 10.1093/gerona/glw240 (2016). [DOI] [PubMed]
- 11.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing research reviews. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasch S, et al. Chronic pancreatitis: Do serum biomarkers provide an association with an inflammageing phenotype? Pancreatology: official journal of the International Association of Pancreatology. 2016;16:708–714. doi: 10.1016/j.pan.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Siriwardena AK, Mason JM, Sheen AJ, Makin AJ, Shah NS. Antioxidant therapy does not reduce pain in patients with chronic pancreatitis: the ANTICIPATE study. Gastroenterology. 2012;143:655–663 e651. doi: 10.1053/j.gastro.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 14.Wan Leung S, et al. Health-related quality of life in 640 head and neck cancer survivors after radiotherapy using EORTC QLQ-C30 and QLQ-H&N35 questionnaires. BMC cancer. 2011;11:128. doi: 10.1186/1471-2407-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inagaki M, et al. Associations of interleukin-6 with vegetative but not affective depressive symptoms in terminally ill cancer patients. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2013;21:2097–2106. doi: 10.1007/s00520-013-1767-x. [DOI] [PubMed] [Google Scholar]
- 16.Meyer T, et al. Serum levels of interleukin-6 and interleukin-10 in relation to depression scores in patients with cardiovascular risk factors. Behavioral medicine. 2011;37:105–112. doi: 10.1080/08964289.2011.609192. [DOI] [PubMed] [Google Scholar]
- 17.Wunsch E, et al. In patients with liver cirrhosis, proinflammatory interleukins correlate with health-related quality of life irrespective of minimal hepatic encephalopathy. European journal of gastroenterology & hepatology. 2013;25:1402–1407. doi: 10.1097/MEG.0b013e328365a447. [DOI] [PubMed] [Google Scholar]
- 18.Komar HM, Hart PA, Cruz-Monserrate Z, Conwell DL, Lesinski GB. Local and Systemic Expression of Immunomodulatory Factors in Chronic Pancreatitis. Pancreas. 2017;46:986–993. doi: 10.1097/MPA.0000000000000896.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olesen SS, et al. Pharmacological pain management in chronic pancreatitis. World J Gastroenterol. 2013;19:7292–7301. doi: 10.3748/wjg.v19.i42.7292.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maisonneuve P, et al. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut. 2005;54:510–514. doi: 10.1136/gut.2004.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han S, et al. Quality of life comparison between smokers and non-smokers with chronic pancreatitis. Pancreatology: official journal of the International Association of Pancreatology. 2018;18:269–274. doi: 10.1016/j.pan.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machicado JD, et al. Quality of Life in Chronic Pancreatitis is Determined by Constant Pain, Disability/Unemployment, Current Smoking, and Associated Co-Morbidities. The American journal of gastroenterology. 2017;112:633–642. doi: 10.1038/ajg.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maurer M, von Stebut E. Macrophage inflammatory protein-1. The international journal of biochemistry & cell biology. 2004;36:1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Charrier A, Chen R, Kemper S, Brigstock DR. Regulation of pancreatic inflammation by connective tissue growth factor (CTGF/CCN2) Immunology. 2014;141:564–576. doi: 10.1111/imm.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon NM, et al. A detailed examination of cytokine abnormalities in Major Depressive Disorder. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2008;18:230–233. doi: 10.1016/j.euroneuro.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stuart MJ, Baune BT. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: a systematic review of biomarker studies. Neuroscience and biobehavioral reviews. 2014;42:93–115. doi: 10.1016/j.neubiorev.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Kiguchi N, Maeda T, Kobayashi Y, Fukazawa Y, Kishioka S. Macrophage inflammatory protein-1alpha mediates the development of neuropathic pain following peripheral nerve injury through interleukin-1beta up-regulation. Pain. 2010;149:305–315. doi: 10.1016/j.pain.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 28.Xue, J. et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. - PubMed - NCBI. (2018). [DOI] [PMC free article] [PubMed]
- 29.Shinoda H, et al. Elevated CC chemokine level in bronchoalveolar lavage fluid is predictive of a poor outcome of idiopathic pulmonary fibrosis. Respiration; international review of thoracic diseases. 2009;78:285–292. doi: 10.1159/000207617. [DOI] [PubMed] [Google Scholar]
- 30.Yogo Y, et al. Macrophage derived chemokine (CCL22), thymus and activation-regulated chemokine (CCL17), and CCR4 in idiopathic pulmonary fibrosis. Respiratory research. 2009;10:80. doi: 10.1186/1465-9921-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen LJ, Lv J, Wen XY, Niu JQ. CXC chemokine IP-10: a key actor in liver disease? Hepatology international. 2013;7:798–804. doi: 10.1007/s12072-013-9445-0. [DOI] [PubMed] [Google Scholar]
- 32.Xu Z, Zhang X, Lau J, Yu J. C-X-C motif chemokine 10 in non-alcoholic steatohepatitis: role as a pro-inflammatory factor and clinical implication. Expert reviews in molecular medicine. 2016;18:e16. doi: 10.1017/erm.2016.16. [DOI] [PubMed] [Google Scholar]
- 33.Rea IM, et al. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Frontiers in immunology. 2018;9:586. doi: 10.3389/fimmu.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang M, et al. IL-7 inhibits fibroblast TGF-beta production and signaling in pulmonary fibrosis. The Journal of clinical investigation. 2002;109:931–937. doi: 10.1172/JCI14685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annual review of pathology. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banerjee C, et al. Identification of serum biomarkers for aging and anabolic response. Immunity & ageing: I & A. 2011;8:5. doi: 10.1186/1742-4933-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okafor CN, et al. Body mass index, inflammatory biomarkers and neurocognitive impairment in HIV-infected persons. Psychology, health & medicine. 2017;22:289–302. doi: 10.1080/13548506.2016.1199887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trollor JN, et al. The association between systemic inflammation and cognitive performance in the elderly: the Sydney Memory and Ageing Study. Age. 2012;34:1295–1308. doi: 10.1007/s11357-011-9301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinho AV, et al. Adult pancreatic acinar cells dedifferentiate to an embryonic progenitor phenotype with concomitant activation of a senescence programme that is present in chronic pancreatitis. Gut. 2011;60:958–966. doi: 10.1136/gut.2010.225920. [DOI] [PubMed] [Google Scholar]
- 40.Fitzner B, et al. Senescence determines the fate of activated rat pancreatic stellate cells. Journal of cellular and molecular medicine. 2012;16:2620–2630. doi: 10.1111/j.1582-4934.2012.01573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study, that are not included in this published article (and its Supplementary Information Files), are available from the corresponding author on reasonable request.


