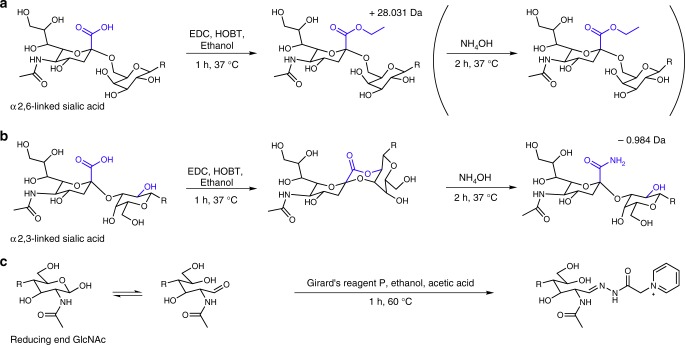
Fig. 1.
Reaction scheme for the linkage-specific derivatization of sialic acids and subsequent GirP labelling. a The α2,6-linked sialic acid forms an ethyl ester during the first step of the reaction and remains stable throughout. b The α2,3-linked sialic acid initially loses water forming a lactone. After the addition of ammonia, the lactone ring is opened and a stable amide is formed. c The reducing ends of all N-glycans are labelled with Girard’s reagent P