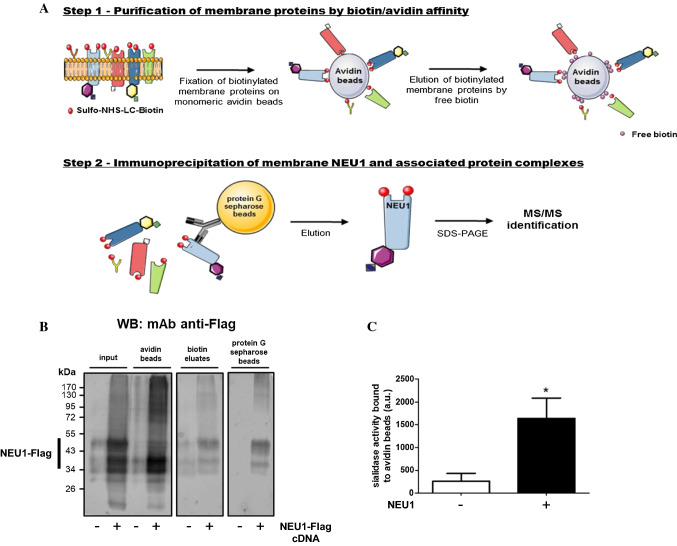
Fig. 1.
Workflow of the proteomic approach and validation of the purification procedure. a The approach uses a two-step purification. The first step involves biotinylation of plasma membrane proteins from adherent COS-7 cells overexpressing NEU1-Flag with the non-permeable reagent EZ-Link® sulfo-NHS-LC-biotin. After cell lysis, protein lysates were incubated with monomeric avidin agarose beads to retain biotinylated plasma membrane proteins. After elution by free biotin, recovered proteins were subjected to the second step of purification which consists of immunoprecipitation of NEU1 and associated interaction partners using polyclonal rabbit anti-NEU1 antibodies. Bound material was then eluted from beads, fractionated by SDS-PAGE and stained by Coomassie blue. After systematic excision from the gel and in-gel trypsin digestion, the extracted peptides were analyzed by nano-LC–ESI MS/MS using an Orbitrap mass spectrometer. b COS-7 cells overexpressing human NEU1-Flag were subjected to the purification procedure (a) and the presence of NEU1-Flag at each step of the purification was checked by Western blot using a mouse monoclonal anti-Flag antibody (1/1000). The figure is representative of three independent experiments. NEU1-Flag−, non-transfected cells; NEU1-Flag+, cells co-transfected with NEU1-Flag and PPCA. c Sialidase activity associated with monomeric avidin agarose beads was measured using 200 µM of 2-O-(p-nitrophenyl)-α-d-N-acetylneuraminic acid substrate in 20 mM MES at pH 4.5. Results are expressed as mean ± SEM of four independent experiments and statistical analysis was performed by Student’s t test (*p < 0.05). NEU1− non-transfected cells; NEU1+ cells co-transfected with NEU1 and PPCA