Abstract
Background
To improve tendon-to-bone healing in anterior cruciate ligament (ACL) reconstruction, a novel technique via the calcium phosphate (CaP) hybridization method for tendon graft using an alternate soaking process was developed. The use of the CaP hybridization method for quadriceps tendon-bone (QTB) graft in ACL reconstruction has not been reported in previous studies. Thus, this clinical trial aimed to clarify the safety and feasibility of using CaP hybridization method for quadriceps tendon-bone (QTB) graft in ACL reconstruction.
Methods
Eight patients (average age, 41.6 ± 10.6 years; 2 men and 6 women) with unilateral ACL injury were included in this study. They underwent ACL reconstruction using QTB graft that hybridized CaP. The follow-up period was from 2 months to 4 years (average, 2.4 ± 1.5 years). Cases of adverse events, including tumor formation, infection, fracture, contracture, severe pain, and re-rupture, were recorded. Moreover, clinical results (KT-1000 arthrometry, pivot-shift test, International Knee Documentation Committee grade, Lysholm scale, and sports activity level), and images of graft and bone tunnel (magnetic resonance imaging, arthroscopic appearance, and computed tomography) were also evaluated.
Results
No adverse events were observed in the follow-up periods. Postoperative clinical results showed improvement compared with the preoperative findings. The sports activity level after the surgery became equivalent to that before injury. There was no progression of bone tunnel enlargement.
Conclusions
Using the CaP hybridization method for QTB graft in ACL reconstruction was safe and feasible in the clinical trial. Moreover, this method may improve clinical outcomes. In the future, it is necessary to verify the effect of the CaP hybridization method for QTB graft in ACL reconstruction.
Keywords: Safety and feasibility, Anterior cruciate ligament reconstruction, Calcium phosphate hybridization, Quadriceps tendon-bone graft, Clinical trial
Abbreviations: ACL, Anterior cruciate ligament; CaP, Calcium phosphate; QTB, Quadriceps tendon-bone; IKDC, International knee documentation committee; MRI, Magnetic resonance imaging; CT, Computed tomography; CSAs, Cross-sectional areas
1. Introduction
To improve tendon-to-bone healing in anterior cruciate ligament (ACL) reconstruction, a novel technique via the calcium phosphate (CaP) hybridization method for tendon graft using an alternate soaking process was developed.1 After ACL reconstruction using a tendon graft, only the fibrous tissue is noted in the grafted tendon–bone interface.2,3 By hybridizing the CaP to the tendon graft, direct bonding between the grafted tendon and the newly formed bone without scar tissue formation at 2–3 weeks after ACL reconstruction was observed in rabbits.4 The tendon graft that hybridized CaP achieved better knee functions (greater in situ forces in the graft and/or anterior knee stability) under applied anterior tibial loads than an untreated tendon graft at 6 months and 1 year after ACL reconstruction in goats.3,5 In a clinical trial, the hamstring tendon graft that hybridized CaP in non-anatomic single-bundle ACL reconstruction improved clinical results and reduced the bone tunnel enlargement compared with that using the conventional method in the 2-year follow-up.6 In anatomic single-bundle ACL reconstruction, the hamstring tendon graft that hybridized CaP was safe, and clinical results at 2 years postoperatively showed improvement compared with the preoperative data.7
In addition to hamstring tendon graft, a quadriceps tendon-bone (QTB) graft is generally used and has achieved good clinical outcomes in anatomic ACL reconstruction.8,9 The patella bone side of the QTB graft is inserted into the femoral bone tunnel, and the quadriceps tendon side of the QTB graft is inserted into the tibial bone tunnel in anatomic ACL reconstruction.9 Therefore, bonding of the graft and bone tunnel is bone to bone on the femoral side, whereas, this is tendon to bone on the tibial side. The use of the CaP hybridization method for QTB graft in ACL reconstruction has not been reported in previous studies. Therefore, this study aimed to present our newly established CaP hybridization method on the quadriceps tendon inserted into the tibial bone tunnel to improve the biological fixation on the tibial side, and to clarify the safety and feasibility of this novel technique through a clinical trial. We hypothesized that using the CaP hybridization method for QTB graft in ACL reconstruction will be safe and feasible and will improve the clinical outcomes.
2. Methods
Between March 2013 and March 2015, 8 patients (average age, 41.6 ± 10.6 years; 2 men and 6 women) who were scheduled to undergo arthroscopically assisted unilateral anatomic ACL reconstruction using a QTB graft that hybridized CaP were included in the present study (Table 1). The follow-up period was from 2 months to 4 years (average 2.4 ± 1.5 years). The ethics committee of our institution reviewed and approved the study (approval number: 1101). Informed consent was obtained from the enrolled patients. We excluded cases using grafted tendons other than QTB, revision cases, multi-ligamentous surgical cases, and bilateral ACL reconstruction cases. All ACL reconstructions were performed by two experienced surgeons.
Table 1.
Patients’ characteristics and operative details.
| Age (years) | 41.6 ± 10.6 (n = 8) |
|---|---|
| Sex (male/female) | 2/6 |
| Height (cm) | 157.8 ± 7.7 (n = 8) |
| Weight (kg) | 55.8 ± 15.7 (n = 8) |
| Duration from injury to operation (months) | 18.6 ± 41.4 (n = 8) |
| Follow-up period (years) | 2.4 ± 1.5 (n = 8) |
| Operative side (right/left) | 4/4 |
| Operative time (min) | 108.4 ± 13.5 (n = 8) |
| Operative findings of meniscal injury (MM/LM) | MM: 5, LM: 2 |
| Operative findings of cartilage injury (medial/lateral) | medial: 2, lateral: 1 |
| Graft length (mm) | 55.9 ± 3.2 (n = 8) |
| Graft diameter of the femoral side (mm) | 8.7 ± 0.5 (n = 8) |
| Graft diameter of the tibial side (mm) | 9.5 ± 0.5 (n = 8) |
Average ± standard deviation.
MM, medial meniscus; LM, lateral meniscus.
2.1. Surgical procedure
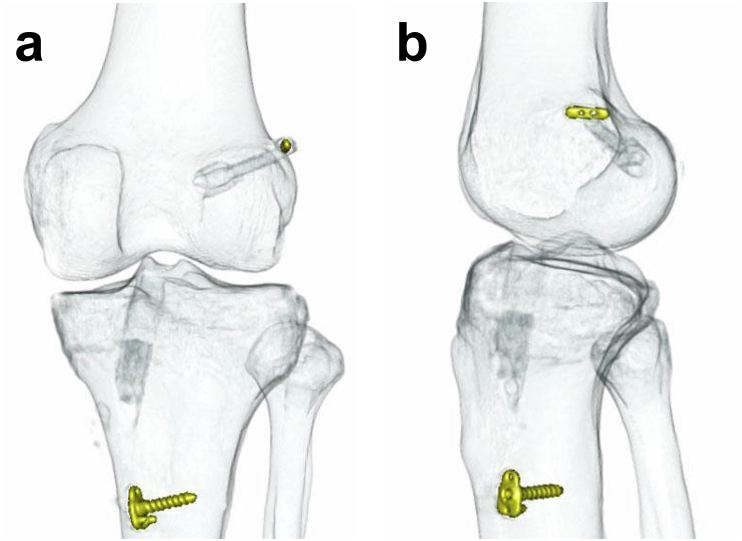
After arthroscopic evaluation and treatment of associated lesions, the QTB graft was harvested. The bone part of the QTB was hooked to the BTB TightRope RT® (Arthrex, Naples, FL, USA) on the femoral side. The tendon part of the QTB was whip-stitched with FiberWire® #2 (Arthrex, Naples, FL, USA) on the tibial side. The length and diameter of the tendon grafts were 50–60 and 8.0–10.0 mm, respectively (Table 1). Both the femoral and tibial bone tunnels were anatomically created at the tibial and femoral insertions of the ACL using the outside-in tunnel technique. An 11- to 15-mm long femoral socket was created according to the length of the bone graft. The tibial bone tunnel was created using a core reamer (Arthrex, Naples, FL, USA), and cylindrical bone was obtained. The graft was fixed on the lateral femoral cortex, and then fixed to a screw and washer on the tibial side with an initial tension of a few Newtons at 0° knee flexion. The harvested cylindrical bone was grafted from the distal tibial bone tunnel after the placement of the QTB (Fig. 1).
Fig. 1.
Three-dimensional computed tomographic image of anterior cruciate ligament reconstruction using quadriceps tendon-bone graft that hybridized calcium phosphate.
(a) Anteroposterior view.
(b) Lateral view.
2.2. Intraoperative calcium phosphate hybridization method
The intraoperative CaP hybridization method was similar to that used in our previous study.6,7,10 After graft preparation, the femoral and intraarticular portions of the tendon graft were covered with the sleeve of a rubber glove tied with nonabsorbable sutures to prevent CaP hybridization. Then, the graft of the tibial side was soaked in a calcium solution (100 mM CaCl2 + 30 mM l-histidine, pH 7.4, 280 mOsm/l, 20 °C) for 30 s. The grafts were subsequently soaked in a NaHPO4 solution (116.4 mM NaH2PO4:128.7 mM Na2HPO4・12H2O = 15%:85%, pH 7.4, 280 mOsm/l, 20 °C) for 30 s. Before each soaking step, the grafts were washed in a saline solution. This cycle was repeated 10 times.6,7,10 The CaP hybridization could be completed within the operative time.
2.3. Postoperative rehabilitation
Postoperative rehabilitation was similar to that performed in our previous study.7,10 After surgery, the knee was immobilized at 20° flexion with a removable postoperative brace for 1 week. Range of motion exercise and partial weightbearing were started at 1 week postoperatively, and full weightbearing walking was allowed at 3 weeks postoperatively. Running was allowed at 3 months postoperatively, and return to sports was allowed after 6–12 months. The postoperative rehabilitation was the same for all patients, even those who underwent partial meniscectomy or meniscal repair.
2.4. Clinical evaluations
Cases of any adverse events, including tumor formation, infection, fracture, contracture, severe pain, and re-rupture, were recorded.
The patients were assessed preoperatively, and at 1, 2, 3, and 4 years postoperatively. Anterior knee laxity was assessed using the KT-1000 arthrometer (MEDmetric, San Diego, CA, USA) at manual maximum anterior tibial load. The pivot-shift test, the Tegner scale,11 the International Knee Documentation Committee (IKDC) grade,12 and the Lysholm scale11 were also used during the evaluation. Preoperative Tegner scale data were assessed using data obtained before ACL injury.
2.5. Magnetic resonance imaging (MRI) evaluation
The MRI evaluation method was similar to that described previously.6,7 MRI evaluation was performed to analyze the intensity of the graft, which is a measure of graft remodeling, at 1 and 2 years postoperatively. The grafts of the mid-substance area were characterized as low intensity, iso intensity, or high intensity by proton density imaging in the sagittal, coronal, and axial planes with 3.0-Tesla MRI (MAGNETOM Verio Dot; Siemens Healthineers, Earlangen, Germany).6,7,13
2.6. Arthroscopic evaluation
The arthroscopic evaluation method was similar to that used in our previous report.6,7 Second-look arthroscopic examination was performed once at 1–2 years postoperatively to analyze the synovium coverage of the graft, which provides an indication of revascularization. The synovium coverage of each reconstructed graft was graded as A (completely covered), B (partially covered), or C (almost uncovered).6,7,14
2.7. Computed tomography (CT) analysis
All patients underwent CT evaluation at 1 week, 1 year, and 2 years postoperatively. CT scans (voltage 80 kV; Activion 16; Toshiba Medical Systems, Otawara, Japan) were performed with the knee in full extension. Initial volume acquisition was made with 2-mm cuts. Three-dimensional images were reconstructed using a Virtual Place Lexus workstation (AZE, Tokyo, Japan).6,7,10 We evaluated the tibial tunnels that used the CaP hybridization method for the tunnel enlargement rates of the cross-sectional areas (CSAs) at the apertures from 1 week to 1 year postoperatively, and from 1 week to 2 years postoperatively, according to the previous studies.6,7,10
The bone tunnel enlargement evaluation was performed using a method similar to that used in previous studies.6,7,10 The tunnels were cut along planes perpendicular to the long axes. We measured the tunnel CSAs of the tibia at the sites closest to the joint aperture. The increase in tunnel CSA was calculated as: CSA increase rate (%) = (CSA at 1 year or 2 year – CSA at 1 week) × 100/CSA at 1 week.
2.8. Statistical analyses
The paired t-test was used to analyze the KT-1000 arthrometry and Lysholm scores between preoperatively and each year postoperatively until 3 years within the same group. The Wilcoxon signed-rank test was used to analyze the pivot-shift test and IKDC results between preoperatively and each year postoperatively until 3 years within the same group. The Wilcoxon signed-rank test was also used to analyze the Tegner scores between preinjury and each year postoperatively until 3 years within the same group. Given that the amount of data for 4 years after surgery was less, statistical analysis could not be performed. Differences were considered significant at P < 0.05.
3. Results
There were no adverse events in the follow-up period. There were no re-rupture cases. The anterior knee laxity at 1, 2, and 3 years postoperatively was significantly lesser than that obtained preoperatively (Table 2). The pivot-shift test and IKDC results at 1, 2, and 3 years postoperatively improved compared with those obtained preoperatively (Table 2). The Lysholm score at 1, 2, and 3 years postoperatively was significantly higher than that obtained preoperatively (Table 2). There was no significant difference between the preinjury and postoperative Tegner scores (Table 2).
Table 2.
Patients’ clinical outcomes.
| KT-1000 arthrometry (mm) | |
|---|---|
| Preoperative | 9.5 ± 2.3 (n = 8) |
| 1 year | 0.9 ± 1.6* (n = 7) |
| 2 years | 0.0 ± 0.0* (n = 5) |
| 3 years | −0.2 ± 0.4* (n = 5) |
| 4 years | −0.5 ± 0.7 (n = 2) |
| Pivot-shift test: 0, 1+, 2+, 3+ (n) | |
| Preoperative | 0, 1, 7, 0 (n = 8) |
| 1 year | 7, 0, 0, 0* (n = 7) |
| 2 years | 5, 0, 0, 0* (n = 5) |
| 3 years | 5, 0, 0, 0* (n = 5) |
| 4 years | 2, 0, 0, 0 (n = 2) |
| Tegner activity score: 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 (n) | |
| Preinjury | 0, 0, 0, 1, 1, 1, 3, 2, 0, 0, 0 (n = 8) |
| 1 year | 0, 0, 0, 2, 0, 2, 2, 1, 0, 0, 0 (n = 7) |
| 2 years | 0, 0, 0, 1, 1, 1, 2, 0, 0, 0, 0 (n = 5) |
| 3 years | 0, 0, 0, 1, 1, 1, 2, 0, 0, 0, 0 (n = 5) |
| 4 years | 0, 0, 0, 1, 0, 0, 1, 0, 0, 0, 0 (n = 2) |
| IKDC objective grade: A, B, C, D (n) | |
| Preoperative | 0, 0, 4, 4 (n = 8) |
| 1 year | 6, 1, 0, 0 (n = 7) |
| 2 years | 5, 0, 0, 0 (n = 5) |
| 3 years | 5, 0, 0, 0 (n = 5) |
| 4 years | 2, 0, 0, 0 (n = 2) |
| Lysholm score | |
| Preoperative | 45.6 ± 11.7 (n = 8) |
| 1 year | 94.4 ± 4.4* (n = 7) |
| 2 years | 97.2 ± 5.2* (n = 5) |
| 3 years | 98.2 ± 3.0* (n = 5) |
| 4 years | 96.5 ± 4.9 (n = 2) |
*Significant difference compared with the preoperative or preinjury value (P < 0.05).
Average ± standard deviation.
IKDC, International Knee Documentation Committee.
The intensity of the graft was iso or low in the MRI evaluation (Table 3). None of the cases had high intensity (Table 3). The arthroscopic appearance was all graded A (Table 3).
Table 3.
Postoperative MRI findings, arthroscopic appearance, and tibial bone tunnel enlargement of cross-sectional area.
| Graft intensity on MRI: low, iso, high (n) | |
|---|---|
| 1 year | 3, 4, 0 (n = 7) |
| 2 years | 3, 2, 0 (n = 5) |
| Arthroscopic appearance: A, B, C (n) | 7, 0, 0 |
| Tibial bone tunnel enlargement of cross-sectional area (%) | |
| 1 year | 9.9 ± 40.9 (n = 7) |
| 2 years | 4.2 ± 25.5 (n = 5) |
The arthroscopic appearance of the synovium coverage of each graft was graded as A (completely covered), B (partially covered), or C (almost uncovered).
*Significant difference between 1 year and 2 years (P < 0.05).
Average ± standard deviation.
MRI, magnetic resonance imaging.
The increased CSA rate in the tibial bone tunnel was not significantly different between 1 year and 2 years postoperatively (Table 3).
4. Discussion
The findings of the present study revealed that anatomic ACL reconstruction using the QTB graft that hybridized CaP was safe and feasible, because no adverse events, such as tumor formation, infection, fracture, contracture, severe pain, and re-rupture, were observed in the follow-up periods. Moreover, the postoperative intensity of the graft in the MRI and arthroscopic appearance were not worse. Furthermore, clinical results improved postoperatively compared with the preoperative data. This is the first clinical trial of the CAP hybridization method for QTB in ACL reconstruction.
Previous studies have demonstrated the safety of using the CaP hybridization method for hamstring tendon graft.6,7 In the hamstring tendon graft, the femoral and tibial sides both undergo CaP hybridization. However, in this study, CaP hybridization was performed only on the tibial side. Therefore, it is considered that the amount of hybridized CaP in the QTB graft in this study was lesser than that in the hamstring tendon graft. Therefore, the influence of the CaP on the QTB graft can be small. The presence of high intensity in the graft during the MRI evaluation indicates a maturation defect.15 Therefore, it is considered that the CaP hybridization method did not cause complications in the grafts on MRI. Moreover, the arthroscopic appearance evaluation showed completed synovium coverage of the graft. Therefore, the MRI and arthroscopic analysis are indicating that the CaP-hybridized tendon graft is safe for use clinically. Moreover, the CaP hybridization could be completed within an operative time, and thus, can be considered to be feasible.
The clinical results were improved postoperatively compared with the preoperative data obtained after the ACL reconstruction. Moreover, the sports activity level of the patients postoperatively was comparable to that before the injury. The postoperative clinical results were consistent with the results of a previous study that utilized the CaP hybridization method for the hamstring tendon graft in anatomic ACL reconstruction.7 Therefore, improvement of clinical results postoperatively can be expected by using the CaP hybridization method for the QTB graft in anatomic ACL reconstruction. However, a comparative study between the CaP hybridization method and the conventional method with a longer follow-up is needed to confirm the clinical effects of the CaP hybridization method for the QTB graft in anatomic ACL reconstruction.
In the present study, bone tunnel enlargement did not progress from 1 year to 2 years postoperatively, similar to the results of the previous study using hamstring tendon graft that hybridized CaP.7 The CaP-hybridized tendon graft enhances bone formation on the surface of the tendon graft in the bone tunnel compared with that in the untreated tendon graft.4 Therefore, more new bones can form in the tibial bone tunnels using the CaP method because of its osteogenic effect. Previous studies using goats revealed that the CaP-hybridized tendon graft enhances tendon-to-bone healing and new formation of bone near the joint aperture of the femoral and tibial bone tunnels.3,5 The biological fixation provided by new bone formation may effectively prevent the progression of bone tunnel enlargement. However, a longer follow-up study is needed to investigate bone tunnel enlargement in the CaP hybridization method for the QTB graft in anatomic ACL reconstruction.
The present study had some limitations. There were missing data, and the follow-up period was short. A study with a longer follow-up and a complete dataset is needed. In the future, it is necessary to prospectively and randomly compare the CaP hybridization method with the conventional method. Moreover, the effect of the CaP hybridization method for QTB graft in ACL reconstruction needs to be verified.
5. Conclusions
Using the CaP hybridization method for QTB graft in ACL reconstruction was safe and feasible in this clinical trial. Moreover, this method may improve patients’ clinical outcomes.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval and consent to participate
The ethics committee of Ichihara Hospital reviewed and approved this retrospective study (approval number 1101).
Authors’ contributions
HM and TK conceived the study and participated in its design and coordination. HM and TK performed the operations. HM analyzed the data and drafted the manuscript. All authors interpreted the data and participated in drafting the text and tables. All authors read and approved the final manuscript.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
Contributor Information
Hirotaka Mutsuzaki, Email: mutsuzaki@ipu.ac.jp.
Tomonori Kinugasa, Email: yan-k@da2.so-net.ne.jp.
Masataka Sakane, Email: sakane-m@tsukuba-seikei.jp.
References
- 1.Taguchi T., Kishida A., Akashi M. Hydroxyapatite formation on/in hydrogels using a novel alternate soaking process. Chem Lett. 1998;8:711–712. [Google Scholar]
- 2.Nebelung W., Becker R., Urbach D., Röpke M., Roessner A. Histological findings of tendon-bone healing following anterior cruciate ligament reconstruction with hamstring grafts. Arch Orthop Trauma Surg. 2003;123:158–163. doi: 10.1007/s00402-002-0463-y. [DOI] [PubMed] [Google Scholar]
- 3.Mutsuzaki H., Sakane M., Fujie H., Hattori S., Kobayashi H., Ochiai N. Effect of calcium phosphate–hybridized tendon graft on biomechanical behavior in anterior cruciate ligament reconstruction in a goat model: novel technique for improving tendon-bone healing. Am J Sports Med. 2011;39:1059–1066. doi: 10.1177/0363546510390427. [DOI] [PubMed] [Google Scholar]
- 4.Mutsuzaki H., Sakane M., Nakajima H. Calcium-phosphate-hybridized tendon directly promotes regeneration of tendon-bone insertion. J Biomed Mater Res A. 2004;70A:319–327. doi: 10.1002/jbm.a.30084. [DOI] [PubMed] [Google Scholar]
- 5.Mutsuzaki H., Fujie H., Nakajima H., Fukagawa M., Nomura S., Sakane M. Effect of calcium phosphate hybridized tendon graft in anatomical single-bundle ACL reconstruction in goats. Orthop J Sports Med. 2016;4 doi: 10.1177/2325967116662653. 2325967116662653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mutsuzaki H., Kanamori A., Ikeda K., Hioki S., Kinugasa T., Sakane M. Effect of calcium phosphate-hybridized tendon graft in anterior cruciate ligament reconstruction: a randomized controlled trial. Am J Sports Med. 2012;40:1772–1780. doi: 10.1177/0363546512449618. [DOI] [PubMed] [Google Scholar]
- 7.Mutsuzaki H., Kinugasa T., Ikeda K., Sakane M. Anatomic single-bundle anterior cruciate ligament reconstruction using a calcium phosphate-hybridized tendon graft: a randomized controlled trial with 2 years of follow-up. J Orthop Surg Res. 2018;13:327. doi: 10.1186/s13018-018-1045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurley E.T., Calvo-Gurry M., Withers D., Farrington S.K., Moran R., Moran C.J. Quadriceps tendon autograft in anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2018;34:1690–1698. doi: 10.1016/j.arthro.2018.01.046. [DOI] [PubMed] [Google Scholar]
- 9.Barié A., Köpf M., Jaber A. Long-term follow-up after anterior cruciate ligament reconstruction using a press-fit quadriceps tendon-patellar bone autograft. BMC Muscoskelet Disord. 2018;19:368. doi: 10.1186/s12891-018-2271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mutsuzaki H., Kinugasa T., Ikeda K., Sakane M. Calcium phosphate-hybridized tendon grafts reduce femoral bone tunnel enlargement in anatomic single-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26:500–507. doi: 10.1007/s00167-017-4657-9. [DOI] [PubMed] [Google Scholar]
- 11.Tegner Y., Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43–49. [PubMed] [Google Scholar]
- 12.Hefti F., Muller W., Jakob R.P., Staubli H.U. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1:226–234. doi: 10.1007/BF01560215. [DOI] [PubMed] [Google Scholar]
- 13.Endele D., Jung C., Becker U., Bauer G., Mauch F. Anterior cruciate ligament reconstruction with and without computer navigation: a clinical and magnetic resonance imaging evaluation 2 years after surgery. Arthroscopy. 2009;25:1067–1074. doi: 10.1016/j.arthro.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Kondo E., Yasuda K. Second-look arthroscopic evaluations of anatomic double-bundle anterior cruciate ligament reconstruction: relation with postoperative knee stability. Arthroscopy. 2007;23:1198–1209. doi: 10.1016/j.arthro.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Howell S.M., Berns G.S., Farley T.E. Unimpinged and impinged anterior cruciate ligament grafts: MR signal intensity measurements. Radiology. 1991;179:639–643. doi: 10.1148/radiology.179.3.2027966. [DOI] [PubMed] [Google Scholar]