Abstract
During embryogenesis, the musculoskeletal system develops while containing within itself a force generator in the form of the musculature. This generator becomes functional relatively early in development, exerting an increasing mechanical load on neighboring tissues as development proceeds. A growing body of evidence indicates that such mechanical forces can be translated into signals that combine with the genetic program of organogenesis. This unique situation presents both a major challenge and an opportunity to the other tissues of the musculoskeletal system, namely bones, joints, tendons, ligaments and the tissues connecting them. Here, we summarize the involvement of muscle-induced mechanical forces in the development of various vertebrate musculoskeletal components and their integration into one functional unit.
KEY WORDS: Musculoskeletal development, Mechanoregulation, Mechanotransduction
Summary: This Review summarizes how muscle-induced mechanical forces influence the morphogenesis and biomechanical integrity of tendon, joint, bone and muscle, and their integration into a functional unit.
Introduction
The revolution in molecular biology and the identification of signaling pathways and gene regulatory networks have focused attention mostly on the role of molecular signals in regulating tissue and organ development. Yet, in recent years it has become clear that mechanical forces are capable of activating and controlling key cellular processes during organogenesis. Many of these mechanoregulation studies have focused on tensional forces generated by the cytoskeleton (Heer and Martin, 2017). These forces, which can be translated into biochemical signals by molecules possessing mechanotransduction capabilities, are transmitted across transmembrane receptors into the extracellular matrix (ECM) and can also reach neighboring cells. These discoveries have resulted in a fresh view of organogenesis as being regulated through reciprocal interactions between mechanical and chemical cues (reviewed by Mammoto and Ingber, 2010).
Although there is no doubt about the importance of such cell-generated mechanical forces in development, this mode of regulation is only part of the story. During development, tissues and organs can be subjected to, and regulated by, forces that are generated exogenously. For example, forces generated by fluid or air flow regulate numerous developmental processes, such as vasculogenesis and angiogenic sprouting (Chouinard-Pelletier et al., 2013; Galie et al., 2014; Kutys and Chen, 2016; Lucitti et al., 2007), lung branching, alveolar cell differentiation and growth of the airway tree (Jesudason et al., 2006; Nelson and Gleghorn, 2012; Schittny et al., 2000), and pronephron morphogenesis in the developing kidney (Vasilyev et al., 2009).
In the case of musculoskeletal development, exogenous forces acting on tendons and the skeleton are generated by muscle contraction. The importance of mechanical signals in musculoskeletal development has also been highlighted by studies linking restricted fetal movement to developmental abnormalities in humans (see Box 1). The involvement of muscle contraction and embryonic movement in musculoskeletal development was first reported over a century ago. In 1901, Curt Herbest wrote: ʻWeber E.H. found, in a newborn calf which had no spinal cord below the cervical region, and no muscle in the posterior half of the body, that the skeletal parts were well developed. Still, the skeletal parts were only half as heavy as normal, and the joints were ankylosed' (Herbest, 1901). Following on from this report and subsequent pioneering work that further established the involvement of mechanical forces in musculoskeletal development (Fell and Canti, 1934; Hamburger and Waugh, 1940), recent efforts have attempted to map the full scope of the contribution of these mechanical signals and discover how they are integrated into the genetic program to regulate various aspects of musculoskeletal development.
Box 1. The importance of mechanical signals in development: links to developmental disorders.
In humans, developmental abnormalities that arise due to paucity of fetal movement underscore the importance of mechanical signals in musculoskeletal development. A number of factors can contribute to the restriction of fetal movement, including neuropathic or connective tissue disorders, muscle abnormalities, or conditions that limit the intrauterine space (Gordon, 1998). An example of such a developmental defect is arthrogryposis, a syndrome characterized by congenital joint contractures. Although early joint development may be normal, insufficient fetal movement results in the formation of excessive connective tissue around the joints. This fixates the joints and aggravates the contractures (Hall, 1985a,b). Mutation in PIEZO2 results in arthrogryposis and Marden-Walker syndrome [MWKS (OMIM 248700)], which is characterized by kyphoscoliosis and joint contractures (Chesler et al., 2016; Coste et al., 2013; McMillin et al., 2014), supporting the involvement of Piezo genes in mechanotransduction. Another example, which is the most common orthopedic problem in newborn children, is developmental dysplasia of the hip (DDH). This syndrome, which is characterized by abnormal positioning of the femoral head within the acetabulum, is mainly the result of the action of abnormal mechanical forces due to limb position, pressure from the womb, or ligament laxity (Shefelbine and Carter, 2004). Fetal akinesia deformation sequence [FADS (OMIM 208150)] is another developmental abnormality caused by restriction of embryonic mobility. This syndrome is characterized by polyhydramnios, intrauterine growth retardation, pulmonary hypoplasia, craniofacial and limb anomalies, multiple joint contractures and short umbilical cord. The severity of this potentially lethal disease depends on the level of restriction, further highlighting the importance of movement for proper development (Hall et al., 1986).
Here, we review these various studies, focusing mainly on the developing musculoskeleton of the mouse limb – an area in which much progress has been made in recent years. We first provide an overview of how the mouse limb musculoskeletal system develops before highlighting how mechanical forces influence each of its developing components. We also expand the discussion to include other examples of musculoskeletal development, from other organs and other species, in which force plays a role. Finally, we highlight future avenues of research as well as challenges for the field.
An overview of murine limb musculoskeletal tissue development
ʻThe skeleton begins as a continuum, and a continuum it remains all life long. The things that link bone with bone, cartilage, ligaments, membranes, are fashioned out of the same primordial tissue, and come into being pari passu, with the bones themselves. The entire fabric has its soft parts and its hard, its rigid and its flexible parts; but until we disrupt and dismember its bony, gristly and fibrous parts, one from another, it exists simply as a ‘skeleton', as one integral and individual whole.' (pp. 712-713, Thompson, 1917)
Limb development in mice is initiated as cells from the lateral plate mesoderm, which later differentiate into bones, joints, tendons, ligaments and other connective tissues, enter the limb bud (reviewed by Tickle, 2015). Shortly thereafter, cells from the ventrolateral lip of the dermomyotome in the somites migrate into the limb bud to form the limb musculature (reviewed by Comai and Tajbakhsh, 2014). During development, these progenitor cells face two main challenges. One is to proliferate and differentiate to form the different tissues composing the musculoskeleton, and the other is to connect these tissues to form an integrated functional system.
Skeletogenesis in the limb is initiated by condensation of a subset of mesenchymal cells that differentiate into chondroprogenitors, which form the cartilaginous anlagen of future limb bones (reviewed by Berendsen and Olsen, 2015). The transcription factor SRY-box 9 (SOX9) is an essential regulator of this process (Bi et al., 1999). As chondrogenesis progresses and the anlagen of the bone shaft are formed, a secondary wave of differentiating mesenchymal cells is recruited to form bone eminences, superstructures that protrude from the bone surface and define the intricate morphology of each bone (Blitz et al., 2013). Next, a tightly regulated sequence of chondrocyte proliferation and differentiation gives rise to growth plates at both the proximal and distal ends of the anlagen. Subsequently, blood vessels invade the cartilage anlagen, introducing bone-building cells termed osteoblasts. These cells deposit bone matrix and ossify the cartilaginous template from the mid-shaft, pursuing the progression of the growth plates (reviewed by Kronenberg, 2003).
Immediately after the formation of the rudimentary appendicular skeleton by chondroprogenitors, the development of synovial joints is initiated. This process divides the continuous anlagen into segments corresponding to future bones. The joints that form between the segments will enable bones to move relative to one another. The first histological indication for joint formation is the appearance of a higher cell density domain called the interzone at the site of the future joint (Mitrovic, 1977). Molecularly, interzone cells lose the expression of chondrocyte-specific genes such as collagen type II (reviewed by Decker et al., 2014). Instead, they express a new set of genes that includes growth differentiation factor 5 (Gdf5), Wnt4 and Wnt9a (Guo et al., 2004; Hartmann and Tabin, 2001; Später et al., 2006; Storm et al., 1994).
Limb myogenesis is initiated as myogenic progenitors expressing paired box 3 (Pax3) delaminate from the somites and migrate into the limb bud under the regulation of c-Met and hepatocyte growth factor (HGF). Once they have reached the limb, they proliferate and start expressing Myf5 and MyoD (Myod1), which are essential for myogenic determination. Next, under the regulation of myogenic regulatory factors (MRFs), myoblast progenitors differentiate into myocytes that fuse into multinucleated myofibers (reviewed by Buckingham et al., 2003; Chal and Pourquié, 2017; Murphy and Kardon, 2011). Patterning of the newly formed myofibers into over 40 limb muscles is mediated by a pre-pattern comprising transcription factor 4 (TCF4)-expressing connective tissue fibroblasts (Kardon et al., 2003; Mathew et al., 2011). Other factors, such as TBX3, TBX4 and TBX5, HoxA11 and HoxD11, which originate from the limb mesenchyme, also participate in muscle morphogenesis and individualization (Colasanto et al., 2016; Hasson et al., 2010; Swinehart et al., 2013). The formation of functional muscle fibers unfolds in two phases to produce primary and secondary fibers (Harris et al., 1989). In mice, the first phase occurs between embryonic day (E) 10.5 and E12.5 and accounts for ∼20% of the muscle mass in the newborn. The remaining 80% is formed during the second phase, which starts at E14.5 and continues until birth. This stage, termed fetal myogenesis, is characterized by continuous growth and maturation of myofibers (Biressi et al., 2007; Stockdale, 1992).
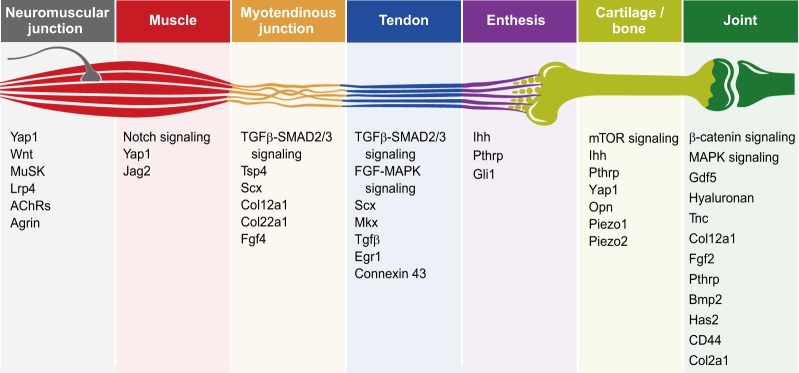
Tendon development is initiated between E11.5 and E12.5 by cells that express the bHLH transcription factor scleraxis (Scx) (Schweitzer et al., 2001). In the limb, this process is regulated by TGFβ signaling (Pryce et al., 2009). Scx+ tendon progenitors are patterned in a loosely organized structure located between differentiating muscles and corresponding cartilage condensations. By E13.5, these rudiments aggregate and differentiate into structurally defined and functional tendons (reviewed by Huang et al., 2015), which are then integrated into muscle-tendon and tendon-skeleton attachments. The formation of muscle-tendon attachments, which are known as myotendinous junctions (MTJs), is mediated primarily by integrins, dystroglycan and other ECM molecules, which form a specialized ECM that differs from that of muscle or tendon. The initial stage of MTJ formation involves secretion of ECM by muscle and tendon cells. Muscles recognize tendons through multiple signals. In zebrafish, thrombospondin (Tsp) and integrin-mediated signaling were shown to mediate tendon-muscle recognition (reviewed by Subramanian and Schilling, 2015). Later during development, contraction-dependent expression of FGF4 at the muscle tip is required for MTJ maintenance and differentiation, whereas tendon cells secrete most of the MTJ ECM (Edom-Vovard et al., 2002; reviewed by Hasson et al., 2017; Valdivia et al., 2017). The establishment of tendon-bone attachments, by contrast, involves a second wave of chondrogenic cells (Blitz et al., 2013). The unique feature of this cell population is that it expresses both the chondrogenic marker Sox9 and the tendon marker Scx (Blitz et al., 2013; Sugimoto et al., 2013). Intriguingly, whereas some of these cells maintain Sox9 but not Scx expression and form bone eminences, others maintain Scx expression while downregulating Sox9 to form tendons. The end result is the in situ formation of a bone eminence that is already connected to a tendon, which will eventually develop into an enthesis.
A clear manifestation of the assembly of these various components – muscle, bone and tendons – into an early functioning musculoskeletal system is the ability of the developing embryo to move. The human fetus starts to move between 8 and 10 weeks of gestation (de Vries et al., 1982). In chick, embryonic movement is observed as early as 4–5 days of incubation, whereas in mouse spontaneous movement is first seen at E12.5. These developmental stages coincide with the establishment of physical contacts between motor axons and presumptive muscle cells (Bekoff, 1981; Bennett et al., 1983; Carry et al., 1983; Hamburger and Balaban, 1963; Martin, 1990; Suzue, 1996) and the formation of neuromuscular junctions (NMJs), which are chemical synapses that form between motor neurons and muscle. Muscle innervation is preceded by the pre-patterning of acetylcholine receptor (AChR) clusters in the muscle. These clusters ultimately define synapse location and, following innervation, are positively regulated by agrin that is secreted from the nerve end. Later, during the first few weeks of life, the number of innervations is decreased, AChR clusters that have not been innervated are lost, and each neuron specifically innervates a single AChR cluster (reviewed by Darabid et al., 2014; Legay and Mei, 2017; Tintignac et al., 2015).
The involvement of mechanical forces in endochondral bone formation
Bone morphology can be regulated at different stages of development, from the patterning of mesenchymal cell condensations and the establishment of the cartilaginous template, through to the establishment of the growth plate and its activity, to the shaping of ossified bone by modeling. As we highlight below, these various events can be regulated by mechanical forces (summarized in Fig. 1).
Fig. 1.
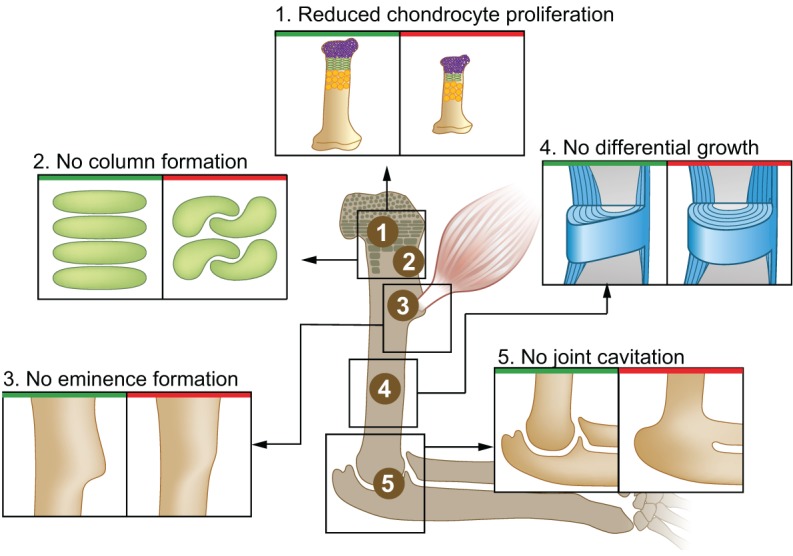
Mechanical forces involved in endochondral bone formation. Bone morphology is regulated by mechanical forces at different levels, as demonstrated by the various developmental and functional aberrations that arise in the absence of muscle contraction. (1) Bone elongation is impaired due to reduced chondrocyte proliferation in the growth plate. (2) Additionally, the organization of resting chondrocytes into columns is impaired, which can also affect skeletal elongation. (3) Bone eminence growth is arrested, resulting in smaller or absent eminences. (4) Differential appositional growth is lost, resulting in a circular circumferential shape. (5) Joint formation is impaired during embryonic development, leading to joint fusion.
The ability of mechanical signals to regulate chondrocyte proliferation was initially demonstrated in three-dimensional cultures of primary chondrocytes (Wu and Chen, 2000; Wu et al., 2001). In vivo, it was shown that cyclic mechanical stimulation of rabbit premaxillae can accelerate chondrocyte proliferation above the normal rate (Wang and Mao, 2002). These effects on chondrocyte proliferation could be mediated by yes-associated protein 1 (YAP1), a mechanosensor that is part of the Hippo signaling pathway. Indeed, changes in YAP cellular localization in chondrocytes were identified in vitro in response to matrix stiffness (Zhong et al., 2013), and YAP was shown to regulate bone size, promote chondrocyte proliferation and inhibit chondrocyte differentiation in vitro and in vivo by suppressing collagen type X (Deng et al., 2016). Moreover, it was recently shown that members of the Hippo pathway are downregulated in muscleless limbs in mice (Rolfe et al., 2014).
In line with these studies, numerous reports have suggested that mechanical load plays a major role in determining bone size. In paralyzed chick and mouse embryos, various cartilaginous skeletal elements are shorter than normal (Gomez et al., 2007; Hall and Herring, 1990; Hamburger and Waugh, 1940; Hosseini and Hogg, 1991; Nowlan et al., 2008, 2010; Pa and Pai, 1965; Rodríguez et al., 1992; Rot-Nikcevic et al., 2006). Studies in chick embryos paralyzed by decamethonium bromide reported a reduction in the size of the proliferative zone, as well as in the number of proliferating chondrocytes (Germiller and Goldstein, 1997; Roddy et al., 2011a). A recent study in West African dwarf crocodiles and in chicks reported that movement regulates chondrocyte proliferation but only in specific growth plates, as the manipulation of movement led to alterations in the proportions between bones of the same limb (Pollard et al., 2017). In that study, differential activation of the mTOR pathway in growth plates was suggested as the underlying molecular mechanism. Differential effects of mechanical load on individual growth plates may not only influence limb proportions. Previously, it was shown that different bones exhibit a specific and unique balance between proximal and distal growth rates to regulate the relative position of superstructures along the bone (Stern et al., 2015). Therefore, modulating the activity of individual growth plates by mechanical forces can lead to changes in the position of bone eminences and, thus, affect bone morphology directly.
Other molecular players that may regulate bone size in response to mechanical cues are Indian hedgehog (IHH) and parathyroid hormone-related protein [PTHrP; also known as parathyroid hormone-like peptide (PTHLH)], which form a negative-feedback loop that regulates chondrocyte proliferation and differentiation (Vortkamp et al., 1996). The genes encoding these factors were shown to respond to mechanical load in the growth plate. For example, IHH expression in chondrocytes is induced by cyclic mechanical stress, while Ihh expression is significantly downregulated in immobilized fetal jaws (Jahan et al., 2014; Rais et al., 2015; Wu et al., 2001). The IHH mechanoresponse is thought to be mediated through primary cilia; in chick, mechanical load was shown to induce ciliogenesis in growth plate chondrocytes, resulting in altered activation of the IHH-PTHrP signaling loop and reduced proliferation (Rais et al., 2015).
In addition to regulating bone length, muscle load was shown to regulate the development of bone eminences. In the absence of muscle activity, bone eminences are significantly smaller or completely lost (Blitz et al., 2009; Gomez et al., 2007; Hamburger and Waugh, 1940; Nowlan et al., 2010; Pa and Pai, 1965; Rot-Nikcevic et al., 2006). Muscular dysgenesis (mdg) mice, which have a mutation in Cacna1s and lack excitation-contraction coupling, leading to paralysis (Pa and Pai, 1965), exhibit complete arrest of chondrocyte proliferation in the ʻmini' growth plate of developing bone eminences, resulting in their loss (Blitz et al., 2009). Collectively, these studies demonstrate that local changes in the mechanical environment can induce local changes in chondrocyte proliferation and, thereby, affect bone morphogenesis.
The organization of resting chondrocytes into columns of discoid proliferative cells, which facilitates skeletal elongation and contributes to skeletal morphogenesis, can also be modulated by mechanical load. Column formation initiates by cell division, where daughter chondrocytes undergo planar division while establishing a cell-cell adhesion surface between them. Next, the cells spread and expand their adhesion surface until they become perpendicular to each other (Romereim et al., 2014). The end result resembles convergent extension, a well-studied morphogenetic process whereby changes in cell organization affect tissue and organ shape. Studies of craniofacial development in zebrafish mutants lacking neuromuscular nicotinic receptors (nic; chrna1), myf5/myod double morphants, in which muscular development is inhibited, or tricaine-paralyzed embryos identified abnormal chondrocyte organization due to failed column formation, resulting in aberrant bone morphology (Shwartz et al., 2012). Interestingly, the effect of paralysis on column formation in the growth plates of mouse embryos was less pronounced, perhaps reflecting the complexity of skeletal morphology in higher vertebrates and suggesting that additional control levels have emerged during evolution.
The distribution and composition of ECM represents another level of mechanical control over skeletal morphogenesis and biomechanical integrity, namely by determining the spacing among cells. Studies in paralyzed chicks and in chondrocytes cultured under loading have shown that mechanical forces can regulate both the content and the dynamics of proteoglycan and collagen production by these cells (Mikic et al., 2004; Wong et al., 2003). Additionally, transcriptomic analyses of humeri from muscleless limbs of mouse embryos revealed that integrins, cadherins and other proteins that associate with the ECM are downregulated (Rolfe et al., 2014).
Another aspect of skeletal morphology is the width and circumferential outline of the bone. Bones grow in width by preferential periosteal growth, which involves repetitive steps of strut-and-ring construction by mineral deposition. Interestingly, different sectors of the bone circumference grow at different rates, resulting in a non-uniform cross-section. Eventually, after endosteal resorption, each bone acquires its typical circumferential shape (Sharir et al., 2011). Several studies have demonstrated the involvement of mechanical load in the mineralization processes that determine bone width and circumference. For example, a study on curare-paralyzed rat fetuses reported alterations in appositional growth of the femur, manifested by smaller and rounder cross-sections (Rodríguez et al., 1992). Myf5/MyoD-deficient mice also exhibit changes in femoral cross-sections, namely increased width and cortical thickness that result in a rounder bone (Gomez et al., 2007). Similar findings are observed in paralyzed mdg mice, where preferential periosteal growth is lost, resulting in a circular circumferential shape (Sharir et al., 2011). Interestingly, finite element analysis showed that bones that had lost the typical circumferential morphology were also mechanically inferior (Sharir et al., 2011), again demonstrating the positive-feedback loop between muscle force and bone morphology and function during development. An interesting candidate for mediating this feedback between mechanical cues and bone modeling is osteopontin (Opn; also known as Spp1), which is considered to be an inhibitor of bone mineralization. Opn was shown to be upregulated in response to mechanical stress in adult mouse teeth (Terai et al., 1999) and downregulated in muscleless limbs of mouse embryos (Rolfe et al., 2014). Additionally, mice lacking Opn do not display a reduction in bone mass in response to muscle unloading (Ishijima et al., 2002).
While the above-mentioned studies reveal an essential role for mechanical forces in bone development, the factors that transduce these forces into biological outcomes are unclear. To date, numerous candidates have been suggested to transduce mechanical signals during development (reviewed by Mammoto and Ingber, 2010; Mammoto et al., 2013). One interesting possibility is presented by the recent finding of two non-selective mechanosensitive cationic channels, PIEZO1 and PIEZO2, which are expressed in a variety of tissue types, including red blood cells, sensory neurons, auditory hair cells, endothelial and epithelial cells, and also in chondrocytes (Florez-paz et al., 2016; Lee et al., 2014; reviewed by Parpaite and Coste, 2017). Their ability to transduce Ca2+ signaling upon mechanical stimulation suggests that they could participate in the response of cartilage to mechanical signals (Lee et al., 2014). However, further studies including in vivo animal models are needed to establish this notion. Another intriguing possibility is that the sensory system, which innervates the bone surface, is involved in the transduction of mechanical signals. In mice, sensory TrkA (Ntrk1)-expressing neurons innervate the developing bone already at the onset of endochondral ossification and, upon mechanical stimulation, osteoblasts secrete nerve growth factor (NGF) to activate an unknown feedback mechanism, which induces bone formation (Tomlinson et al., 2016).
Mechanical control of skeletal configuration
In addition to its involvement in the shaping of individual bones, muscle-induced mechanical load regulates the 3D organization of skeletal elements. An interesting example for such a mechanism comes from birds, in which embryonic muscle activity was shown to be necessary for the rotation and opposability of digit 1, which is used for perching. In the absence of muscle contraction, the digit fails to rotate and acquires a morphology that largely resembles that of the dinosaur ancestor, which probably lacked the opposable thumb function (Francisco Botelho et al., 2015).
Another example of regulation of skeletal arrangement that is affected by muscle load is the patella, also known as the kneecap, the most well-known and studied sesamoid bone (a bone embedded in a tendon). Similarly to bone eminences, the patella develops from a population of Sox9+ and Scx+ progenitors that is initially located on the femur, from which it is later separated by joint formation (Eyal et al., 2015). Similarly to other joints, the patellofemoral joint is regulated mechanically. In the absence of muscle load, the cavitation process fails and the patella remains connected to the femur (Eyal et al., 2015).
The effect of muscle force on skeletal morphology and organization is not restricted to the extremities. Recently, it was shown in chick embryos that prolonged rigid paralysis impairs vertebrae formation, segmentation and spine curvature (Rolfe et al., 2017). In another study, it was reported that muscles and the proprioception system that regulates their activity are necessary to maintain spinal alignment postnatally (Blecher et al., 2017a). Striated muscles and MTJs contain proprioceptive mechanosensors termed muscle spindle and Golgi tendon organ (GTO), respectively (Kokkorogiannis, 2004; Zelená and Soukup, 1983). These organs sense the biomechanical environment, namely changes in muscle length or tension, initiate a rapid neural response through specialized sensory afferent fibers and, ultimately, modulate local muscle tension, thus forming local monosynaptic reflex arcs (Chen et al., 2003; Granit, 1975; Maier, 1997; Moore, 1984). Another example of the involvement of muscles and the proprioceptive system in regulating skeletal morphology is the realignment of fractured long bones. Fractured humeri of mice were shown to realign spontaneously by movement of the two fracture fragments, a process termed natural reduction (Rot et al., 2014). In the absence of either contracting skeletal muscles or functional proprioceptive mechanosensors, the fractured bones fail to realign (Blecher et al., 2017b). Together, these findings suggest a new physiological role for proprioception in non-autonomous regulation of skeletal morphology.
Muscle forces are also involved in musculoskeletal configuration by controlling the positioning of muscles during mouse limb development. In mice, the flexor digitorum superficialis (FDS) muscles, which control paw motion, are located in the forearm; yet, during development they initially form in the paw. As development proceeds, the muscles relocate to their final position in the arm and, interestingly, it was found that muscle contraction is necessary for this translocation (Huang et al., 2013).
The effects of mechanical forces on joint development
As in the case of bone development, a number of studies have revealed that muscle-generated forces can modulate the development of joints (Fig. 1). Studies of joint development in paralyzed chick embryos provided the first indications for the involvement of such forces (Drachman and Sokoloff, 1966; Fell and Canti, 1934; Hamburger and Waugh, 1940; Hosseini and Hogg, 1991; Kahn et al., 2009; Lelkes, 1958; Mikic et al., 2000a; Mitrovic, 1982; Murray and Drachman, 1969; Nowlan et al., 2010; Osborne et al., 2002; Pai, 1965; Persson, 1983; Rot-Nikcevic et al., 2006; Ruano-Gil et al., 1978, 1985). These studies revealed that, in the absence of muscle contraction, multiple joints in the forelimbs and hindlimbs become fused. This effect was also observed in the back, neck and head joints. Later, the same effect was demonstrated in mutant mouse strains in which muscles either fail to form or lack contractility (Kahn et al., 2009; Nowlan et al., 2010; Pai, 1965; Rot-Nikcevic et al., 2006). Impaired development of the jaw joint has also been observed in paralyzed zebrafish embryos (Brunt et al., 2015).
The process of joint development consists of several sequential steps of specification, interzone formation, cavitation and morphogenesis. Studies in chick and mouse have shown that muscle contraction is dispensable for determining the site at which the joint will form, as well as for the induction of joint-forming progenitor cell fate, as evidenced by interzone formation in paralyzed organisms (Drachman and Sokoloff, 1966; Kahn et al., 2009; Mikic et al., 2000b; Mitrovic, 1982). Thus, the first involvement of muscle contraction in joint development is most likely immediately after interzone establishment and before cavitation begins, as muscle contraction at this stage was shown to be necessary for maintaining joint progenitor cell fate; in its absence, progenitors lose their fate and become chondrocytes, eventually resulting in joint loss (Kahn et al., 2009).
Muscle contraction and fetal movement also play a role during the stage of joint morphogenesis (Bastow et al., 2005). The shape of joints has a direct effect on the motility of the organism; thus, the existence of a positive-feedback loop between motility and joint morphology during development is an attractive hypothesis, which might also provide a mechanistic explanation for the adaptation of articular shape to load throughout life. This hypothesis predicts that specific modes of movement would result in distinct joint morphologies. Several studies support this possibility, for example by demonstrating in silico that a combination of an initial joint morphology and a specific movement regime will produce a distinctly shaped joint (Giorgi et al., 2015; Rolfe et al., 2017). This notion is further supported by experimental data showing that imbalance in muscle loading may lead to abnormally shaped joints (Brunt et al., 2016; Ford et al., 2017; Nowlan et al., 2014). A feedback loop between movement and joint shaping could play a central role in the evolutionary adaptation of the skeleton. During evolution, new movement patterns facilitated by morphological changes may prove advantageous, while existing patterns may become superfluous. Cetacean evolution provides an example of the loss of specific muscles and corresponding joints that have become unnecessary: during the limb-to-fin transition, the tricep muscles have undergone atrophy and the elbow joint has been lost (Cooper et al., 2007).
Joint morphogenesis might also be regulated by differential cell proliferation during cartilage growth. Indeed, changes in the mechanical environment of the knee joint were shown to lead to morphological alterations that correlate with regional changes in proliferation (Roddy et al., 2011a,b). This notion is supported by mathematical models of stress distribution, which may modulate growth of the cartilage anlagen to create congruent articular surfaces (Heegaard et al., 1999). Recently, it was reported that the interzone is constantly supplied with new cells, which contribute differentially to developing joint tissues (Shwartz et al., 2016). These findings raise the possibility that muscle contraction might regulate the dynamic behavior of interzone cells.
Interestingly, not all joints are lost in mutant embryos lacking functional muscles. In mice, for example, the knee and the finger joints remain intact (Kahn et al., 2009), whereas in paralyzed chicks the knee joint is lost (Persson, 1983; Roddy et al., 2011a). Explaining this variation is not easy. One plausible explanation is that, in some joints, the lack of muscle-induced signals is compensated for by other components of the joint development genetic program. Indeed, there is evidence that different joints are subject to different modes of regulation. For example, deletion of Tgfbr2 in early limb mesenchyme results in interphalangeal joint fusion without affecting other joints, such as the elbow, despite its high and specific expression in these joints (Seo and Serra, 2007; Spagnoli et al., 2007). Similarly, although Gdf5 is expressed in all synovial joints, only a subset of joints, such as carpal, certain phalangeal and tarsals, are disrupted by Gdf5 null mutations (Storm and Kingsley, 1996). Thus, identifying potential joint-specific mechanisms of regulation and how these might be differentially regulated by muscle contraction is key to gaining a better understanding of joint morphogenesis.
Another key and open issue is the mechanism that responds to mechanical signals in the joint and translates them into molecular signals. One attractive candidate is the β-catenin signaling pathway, the role of which in regulating several aspects of joint development, including maintenance of joint-forming progenitor cell fate, has been firmly established (Guo et al., 2004; Hartmann and Tabin, 2001; Später et al., 2006). Moreover, studies in Drosophila, mouse and, more recently, zebrafish have shown that activation of the β-catenin pathway is dependent on muscle activity (Brunt et al., 2017; Desprat et al., 2008; Kahn et al., 2009). Another signaling pathway that is potentially involved is the MAP kinase (MAPK) pathway, which was shown in chick to be responsive to muscle contraction (Bastow et al., 2005). Additionally, studies in paralyzed chick and mouse embryos have identified numerous factors that are markedly reduced in expression in and around developing joints, including hyaluronic acid (HA) (Bastow et al., 2005; Osborne et al., 2002), tenascin C (Tnc) and collagen type XII (Mikic et al., 2000b), as well as fibroblast growth factor 2 (Fgf2) (Kavanagh et al., 2006), Pthrp, bone morphogenetic protein 2 (Bmp2), hyaluronan synthase 2 (Has2), Cd44, Col2a1 (Roddy et al., 2011a,b), among others (Kahn et al., 2009; Roddy et al., 2011a). However, if and how these factors play a role in contraction-mediated control of joint formation remains an open question.
Mechanical forces and tendon development
Tendons and ligaments are dense connective tissues that coordinate muscle contraction and skeletal movement; whereas tendons attach muscle to skeleton, ligaments attach skeletal elements to each other. Both tissues are composed of fibroblasts, termed tenocytes or ligamentocytes, respectively, which are embedded in a specialized ECM, and both are mechanoresponsive and have the ability to change their composition and arrangement upon changes in mechanical load (Birch et al., 2013; Landis and Silver, 2002; Subramanian and Schilling, 2015; Summers and Koob, 2002; Takimoto et al., 2015).
Tendon development in the limb is a two-stage process. The first stage is mechanical load independent and involves progenitor cell specification, organization and patterning into a densely packed fibrous tissue with a morphology that ranges from broad sheets to highly elastic cables. The bHLH transcription factor Scx, which is the only identified early tendon marker, plays a role in this first step. Scx+ cells give rise to tendons in mouse, chick and zebrafish (Chen and Galloway, 2014; Schweitzer et al., 2001), and Scx positively regulates Col1a1 expression in mouse tendons. However, Scx is not a master regulator of tendon development, and Scx mutant mice are viable and mobile (Schweitzer et al., 2001). Nevertheless, in some tendon groups the progenitors fail to condense upon Scx loss, suggesting that this transcription factor regulates tendon cell adhesion, which is required for their differentiation (Schweitzer et al., 2001; reviewed by Gaut and Duprez, 2016; Schweitzer et al., 2010). The transcription factor mohawk (Mkx) is also implicated in tendon differentiation and regulation, and is able to directly regulate Col1a1 and other tendon structural genes; in postnatal Mkx mutant mice, collagen fibril growth is dramatically reduced and tendons are smaller and hypoplastic (Ito et al., 2010; Liu et al., 2010).
By contrast, the second stage of tendon development in the limb, which involves tendon differentiation, maturation and maintenance, is mechanical load dependent and requires muscle presence and contraction (Fig. 2) (reviewed by Gaut and Duprez, 2016; Kardon, 1998; Schweitzer et al., 2001). Studies performed in mouse, chick and zebrafish embryos have shown that either genetic or surgical muscle inactivation does not affect initial Scx expression in tendon progenitors (Chen and Galloway, 2014; reviewed by Gaut and Duprez, 2016; Havis et al., 2016; Huang et al., 2015; Kardon, 1998; Schweitzer et al., 2001). However, studies performed in chick showed that, in muscleless limbs, autopod tendons degenerate and zeugopod tendons are lost, and under chemical paralysis zeugopod tendon markers are lost (reviewed by Gaut and Duprez, 2016; Kardon, 1998). Interestingly, in mouse, autopod tendons are normal and tendon progenitors are unaffected in muscleless limbs (reviewed by Huang et al., 2015); however, similar to chick, zeugopod tendons are lost. In genetically paralyzed mdg mice, zeugopod tendons are maintained but they are smaller than normal and express less Scx, suggesting that mechanical load has an additional role to that of the muscle tissue itself in tendon development (reviewed by Gaut and Duprez, 2016; Huang et al., 2015). Similarly, it was recently shown that mechanical load in adult mice increases the expression levels of Mkx and other tendon-associated genes in vivo (Kayama et al., 2016).
Fig. 2.
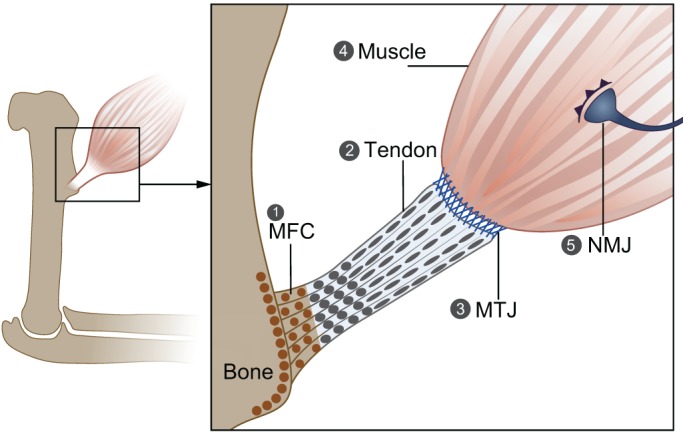
Forces acting during the formation of tendon, muscle and their attachments. The proper formation of tendon, muscle and the attachment between them requires mechanical load. (1) In its absence, mineralized fibrocartilage in the enthesis is lost, and there is increased osteoclast activity and bone resorption in the attachment site. (2) Tendon development is arrested in the absence of muscle, and zeugopod tendons are lost. (3) Proper maturation and the ECM composition of the MTJ, which are regulated by Scx expression, are dependent on muscle contraction. (4) Without muscle contraction, there is a reduction in myotube number, and muscles are smaller than normal and display a delay in splitting. Muscle contraction is also needed to maintain a pool of muscle progenitor cells. (5) In the NMJ, muscle contraction is needed to promote neuronal elimination during development and to prevent NMJ degeneration. MFC, mineralized fibrocartilage; MTJ, myotendinous junction; NMJ, neuromuscular junction.
The TGFβ-SMAD2/3 and FGF-MAPK signaling pathways have also been implicated in tendon mechanotransduction and development. Both were shown to positively regulate Scx, Mkx and other tendon genes in chick and to act independently of one another; the blockage of MAPK signaling in TGFβ gain-of-function chick limbs does not affect positive Scx regulation (Havis et al., 2016). Interestingly, FGF signaling was shown to have opposing effects in chick and mouse models. In chick embryos, FGF signaling is required for the induction of tendon progenitors in the limb, and overexpression of FGF4 from muscle ends was shown to upregulate tenogenesis and Scx expression in response to mechanical load and to rescue Scx expression in muscleless limbs (Edom-Vovard et al., 2002; Havis et al., 2016). In mouse, by contrast, ERK/MAPK inhibition was sufficient to activate Scx in limb mesodermal progenitors and in mesenchymal stem cells (Havis et al., 2014, 2016). The canonical TGFβ signaling pathway, acting through SMAD2/3, is also sufficient to induce Scx expression in stem cells, mouse and chick tendon progenitors and mouse limbs (reviewed by Gaut and Duprez, 2016; Havis et al., 2014; Lorda-Diez et al., 2009; Maeda et al., 2011; Pryce et al., 2009). In the absence of TGFβ signaling, tendon progenitors are specified but are ultimately lost (Pryce et al., 2009). TGFβ-SMAD2/3 signaling was shown to upregulate Scx in an adult tendon culture model in response to mechanical load, and in adult tendons active TGFβ levels were proportional to the extent of tensile load exerted on the tendon (Maeda et al., 2011).
There are two hypotheses for TGFβ activation in response to mechanical load. First, it has been suggested that mechanical force disrupts the structure of the tendon ECM, causing the release of latent TGFβ from the ECM matrix and allowing it to interact with its receptor (reviewed by Subramanian and Schilling, 2015). This interaction leads to an increase in tendon ECM proteins and tendon markers such as Scx, which, in turn, upregulates TGFβ expression. Additionally, TGFβ upregulates matrix metalloproteinases (MMPs), which allows additional release of TGFβ from the ECM. Ultimately, a positive-feedback loop forms by which the ECM adapts to mechanical load (Popov et al., 2015; reviewed by Schweitzer et al., 2010; Subramanian and Schilling, 2015). An alternative mechanism is that other mechanosensitive factors upregulate TGFβ expression in response to mechanical load. One such factor is the early growth response 1 (Egr1) transcription factor, which was identified as a tendon mechanosensitive gene during the muscle-dependent phase of chick and mouse tendon development (Lejard et al., 2011) and was shown to directly regulate TGFβ gene transcription in adult mouse tendons (Gaut et al., 2016; Guerquin et al., 2013). In the absence of EGR1, tendon collagens and matrix proteins are markedly reduced, and EGR1 was shown to upregulate Scx and tenomodulin (Tnmd) in stem cells and Col1a1 in vivo (Guerquin et al., 2013; Lejard et al., 2011).
Finally, it has been suggested that cell-cell communication might play a role in transducing mechanical signals during tendon development. Tenocytes are known to be interconnected via gap junctions, which are multiprotein complexes that undergo assembly or disassembly and mediate cell-cell communication. These junctions are thought to mediate tendon response to mechanical stimulation. In particular, connexin 43 (also known as gap junction protein, alpha 1)-containing gap junctions were shown to inhibit collagen synthesis in response to mechanical load in adult mice, and non-specific gap junction inhibition was shown to suppress the upregulation of collagen synthesis, which is expected to increase in response to mechanical load (Maeda et al., 2012; Waggett et al., 2006).
Force-mediated control of the development of tendon-bone attachments
Owing to large differences in the mechanical properties of tendons and bones, the site at which they attach to one another, termed the enthesis, is considered a weakness point in the musculoskeletal system. Indeed, the attachment between the very elastic tendon and the very rigid bone creates a point of high stress concentration during force transfer, which could lead to detachment (Liu et al., 2014; Thomopoulos, 2011). Dissipation of this stress is achieved either by the formation of fibrous attachments, in which tendon fibers are inserted into the cortical bone in a structure that resembles a root system, or by the formation of a fibrocartilaginous attachment composed of different layers that gradually change in stiffness (Benjamin et al., 2006; Schwartz et al., 2012; Zelzer et al., 2014). Although enthesis development begins in the embryo, the formation of the unique transitional tissue and its subsequent mineralization occur postnatally.
Embryonic development of the enthesis is a two-stage process. First, a specialized pool of cells coexpressing Sox9 and Scx are specified by TGFβ signaling between the cartilage anlagen and the developing tendon (Blitz et al., 2013; Sugimoto et al., 2013). These progenitors are unaffected by mechanical cues and, ultimately, give rise to the enthesis and the bone eminence. By contrast, subsequent development of the bone eminence is regulated by the tendon and is mechanically sensitive. For example, bone eminence cell proliferation and growth depend on muscle contraction, as eminences are lost in both muscleless and paralyzed mice and in paralyzed chick (Hall and Herring, 1990; Hosseini and Hogg, 1991; Pa and Pai, 1965; Rot-Nikcevic et al., 2006). However, the mechanism that transduces the mechanical signal during bone eminence cell proliferation has not been discovered. Additionally, an enthesis still forms in the absence of mechanical signals and eminence formation, although its functionality has not been determined (Blitz et al., 2009).
It was recently shown that during postnatal enthesis development, Hedgehog (Hh)-responsive cells, identified by GLI-Kruppel family member GLI1 (Gli1) expression, give rise to the fibrocartilage enthesis, specifically to its mineralized portion (Dyment et al., 2015; Schwartz et al., 2015), and that in the absence of Hh signaling in Scx+ cells the mineralized fibrocartilage is severely decreased (Breidenbach et al., 2015; Dyment et al., 2015; Liu et al., 2013; Schwartz et al., 2015). Hh signaling is considered a mechanosensitive pathway (Jahan et al., 2014; Rais et al., 2015; Wu et al., 2001). In line with this, it has been demonstrated that botox-induced paralysis results in an increase in Gli1+ cells in the fibrocartilage enthesis, along with reduced mineralized enthesis fibrocartilage, suggesting that mechanical signals regulate the function of Gli1+ cells (Schwartz et al., 2015). In several other studies, botox-induced paralysis also caused increased osteoclast activity and bone resorption, and the formation of disorganized fibrocartilage in the mouse supraspinatus enthesis (Thomopoulos, 2011; Thomopoulos et al., 2010).
Postnatal formation of fibrous entheses is also regulated by PTHrP. In mice, ablation of Pthrp in Scx+ cells results in abnormal fibrous enthesis formation (Wang et al., 2013). Additionally, Pthrp expression was shown to be regulated mechanically in fibrous entheses (Chen et al., 2007). Muscle unloading results in a dramatic decrease in PTHrP levels, suggesting a mechanistic explanation for the ability of muscle load to regulate the development of these entheses. PTHrP and IHH were also shown to co-regulate bone elongation through the formation of a negative-feedback loop, and both were shown to be mechanosensitive (Broadus et al., 2007; Vortkamp et al., 1996; Wu et al., 2001). However, it is still unclear whether these two factors interact during enthesis development and mediate the response to mechanical signals, or whether they act independently or in different types of entheses.
The influence of forces on muscle development
The effect of muscle contraction on the development of muscle itself has been largely neglected. Nonetheless, some recent studies have shown that, similar to the development of other musculoskeletal tissues, the initial specification of myoblasts is independent of mechanical cues (reviewed by Lemke and Schnorrer, 2017), whereas during subsequent stages of development mechanical signals originating from the developing muscle units are needed for proper muscle formation.
During myofiber formation, the early attachment of myotubes to a tendon results in passive tension. This tension, which is needed for the proper assembly and alignment of myotubes during their formation, is suggested to be maintained through titin, a protein that resembles a spring extending half the length of the sarcomere (reviewed by Gautel and Djinovic-Carugo, 2016; Lemke and Schnorrer, 2017; Valdivia et al., 2017). It has also been shown that, in Drosophila, mechanical tension and spontaneous muscle twitching precede the formation of immature muscle fibers (Weitkunat et al., 2014, 2017).
Subsequently, mechanical signals are needed for muscle morphogenesis and mechanical adaptation. In the absence of muscle contraction, muscles are smaller and display a delay in splitting, whereas exercised muscles become larger (de Lima et al., 2016; reviewed by Lemke and Schnorrer, 2017). Additionally, in paralyzed mdg mice, a reduction in myotube number and muscle striation is observed (Pa and Pai, 1965). Mechanical signals also regulate the maintenance of embryonic muscle progenitors. It was previously shown that strain drives the differentiation of mesenchymal stem cells into myoblasts in vitro (De Lisio et al., 2014; reviewed by Valdivia et al., 2017) and that muscle contraction is necessary to maintain the pool of muscle progenitor cells in chick embryos (de Lima et al., 2016). The transcription factor YAP1 was suggested to play a role in this latter context; rigid paralysis in chick reduces the activity of YAP, which was shown to positively regulate jagged 2 (JAG2), a component of the Notch signaling pathway. Under immobilization, YAP, and consequently JAG2, are downregulated, causing a reduction in the number of muscle progenitors and in muscle size and an increase in muscle differentiation (de Lima et al., 2016).
Mechanical forces and the development of muscle-tendon attachments
Muscle force is transferred to the tendon through the MTJ, which is thought to form in a two-stage process. The first stage is independent of mechanical load. During this stage in zebrafish, myoblasts secrete an ECM that is rich in integrin ligands such as Tsp and laminin. Tsp then facilitates the organization of a stable matrix in the MTJ, which is needed for muscle anchoring and muscle-tendon recognition (Subramanian and Schilling, 2014). Accordingly, tsp4 depletion in zebrafish causes muscle detachment upon contraction due to reduced integrin signaling in the ECM (Subramanian and Schilling, 2014; reviewed by Subramanian and Schilling, 2015).
During the second stage, mechanical load from myoblasts triggers Scx expression in tendon progenitor cells through the regulation of TGFβ-SMAD3 signaling. Scx, in turn, regulates the expression of collagens in the tendon and in the MTJ ECM, and facilitates MTJ maturation (Maeda et al., 2011; reviewed by Subramanian and Schilling, 2015). Both COL22 and COL12 play important roles in MTJ integrity under tension. Whereas Col22 was shown to maintain muscle-tendon attachment in zebrafish (Charvet et al., 2013), it was shown in chick that COL12 interacts with TNC and decorin (DCN) to crosslink other collagens during fibril maturation (Veit et al., 2006). Both of these collagens have been implicated in myopathies and tendinopathies in humans (reviewed by Subramanian and Schilling, 2015). Additionally, FGF4 secreted from muscle tips was shown to maintain Scx and Tnc expression in chick tendons (Edom-Vovard et al., 2002; reviewed by Hasson et al., 2017). As the MTJ matures, tenocytes secrete most of the MTJ ECM proteins and adjust its composition. This allows the MTJ to effectively transfer muscle load and prevent muscle-tendon detachment (reviewed by Shwartz et al., 2013; Subramanian and Schilling, 2015).
Mechanical forces and NMJ development
The NMJ is a highly specialized chemical synapse that forms between a motor neuron and a muscle (Fig. 2). Spinal motor neurons innervate individual muscles in order to transmit nerve impulses from neurons to muscle fibers, under the regulation of the central nervous system (reviewed by Legay and Mei, 2017; Tintignac et al., 2015), whereas proprioceptive sensory neurons convey information about the state of the muscle to central neurons through monosynaptic connections between sensory and motor neurons (reviewed by Chen et al., 2003). Importantly, impairments and pathologies that affect NMJs, which can be congenital, autoimmune or due to toxins, can lead to loss of synapses, nerve degeneration or impaired myelination of the nerves, eventually resulting in paralysis or muscle weakness (reviewed by Tintignac et al., 2015).
NMJ formation consists of two stages. In the first, which is nerve independent, a postsynaptic domain is formed in the muscle through a process called pre-patterning. The pre-patterned postsynaptic domain is composed of synaptic muscle-specific kinase (MuSK), low density lipoprotein receptor-related protein 4 (LRP4) and rapsyn proteins, which together form a complex that induces AChR clustering (Lin et al., 2001; Yang et al., 2001). In the second, nerve-dependent stage, agrin secreted from the nerve end binds to LRP4 and MuSK, and mediates further differentiation and maturation of the synapse (reviewed by Legay and Mei, 2017; Tintignac et al., 2015).
Numerous pieces of evidence indicate that innervation affects myotube maturation and muscle development. Innervation can affect the type of muscle fibers or their contractile properties by regulating the expression pattern of muscle-specific genes (reviewed by Tintignac et al., 2015; Washabaugh et al., 1998, 2007). Additionally, AChRs were shown to be required for the induction of spontaneous contraction during embryogenesis (Jaramillo et al., 1988). As for the effect of muscle contraction on NMJ development, it has previously been shown that temporal disuse and muscle paralysis cause nerve and NMJ degeneration in adult animals (Fahim, 1989), and that active muscles are needed for proper neuronal elimination during chick development (Pittman and Oppenheim, 1979). Paralyzed mdg mice exhibit excessive intramuscular nerve branching and increased numbers of ACh and AChR clusters per myofiber; similar phenotypes are seen in chronically paralyzed mouse embryos (Oppenheim et al., 1986; Pinçon-Raymond and Rieger, 1982). Additionally, muscle contraction was shown to promote expression of AChRs after denervation (Lømo et al., 1985).
Interesting new candidates for mechanical signal transduction during NMJ development have recently been discovered. One of these is YAP, the knockout of which in mouse muscles results in smaller and more broadly distributed AChR clusters, many of which are not covered by nerves, unlike in wild-type mice (Zhao et al., 2017). These mice also displayed a decrease in β-catenin in the cytoplasm and nucleus, which suggests that YAP regulates, at least in part, Wnt signaling in the NMJ during its formation. A growing body of evidence implicates the Wnt signaling pathway in NMJ formation (Barik et al., 2014) and, more recently, in the mechanical control of NMJ development. WNT4, WNT9A and WNT11 were shown to enhance AChR clustering in cell culture (Zhang et al., 2012), whereas Wnt4 and Wnt11 initiate muscle pre-patterning in zebrafish (Gordon et al., 2012). Moreover, it was recently shown that WNT4 and WNT11 enhance motor axon outgrowth and AChR clustering in mice (Messéant et al., 2017).
Conclusions
ʻWe see, dimly perhaps, but yet with all the assurance of conviction, that between muscle and bone there can be no change in the one but it is correlated with changes in the other; that through and through they are linked in indissoluble association; that they are only separate entities in this limited and subordinate sense, that they are parts of a whole which, when it loses its composite integrity, ceases to exist.' (pp. 713-714, Thompson, 1917)
In this Review, we have attempted to highlight the main concepts and recent findings on the mechanobiology of musculoskeletal development, focusing on the influence of forces exerted by muscle contraction on neighboring tissues (summarized in Fig. 3). The evidence cited here clearly establishes that muscle forces regulate the development of all the components of the musculoskeletal system. Interestingly, in most cases these forces are not involved in the initial stages of progenitor specification and early patterning; yet, their effect is later necessary for progenitor proliferation, differentiation and for tissue morphogenesis. This muscle-dependent stage of musculoskeletal development initiates a self-amplifying positive-feedback loop between the developing tissues and the mechanical forces. Once the forming tissues become functional, their coordinated activity generates mechanical forces and structured movements that further regulate musculoskeletal development and assembly, as well as functional adaptation of coordinated movement. Nevertheless, future studies might still uncover some form of mechanical regulation during the initial stages of musculoskeletal development.
Fig. 3.
Mechanically regulated signaling pathways, factors and genes involved in musculoskeletal system development. The key signaling pathways, factors and genes, as mentioned in this Review, which have been shown to be regulated by mechanical forces during musculoskeletal development.
Despite the progress that has been made in recent years, a number of challenges still lie ahead. In particular, we lack information about the way that mechanical signals are transformed into molecular signals and biological outputs. This information is crucial not only for a comprehensive understanding of these developmental processes, but also for the development of new therapeutic approaches for treating human diseases related to abnormal musculoskeletal development. In addition, much of our knowledge has come from experiments in in vivo models in which muscles were inactivated either genetically or chemically. Notwithstanding the enormous value of this approach, the ʻall-or-nothing' situation has prevented the study of specific patterns of movement and force, which might be essential for proper development.
To tackle these challenges, it will be necessary to increase the resolution of the analyses. More detailed and accurate mapping of spatiotemporal patterns of force on the one hand, and gene expression on the other, will allow for better correlation between the two. This requires the development of new techniques for manipulating muscle activity, including temporal or partial inhibition as well as the inactivation of single muscles. This approach will provide important information regarding, for example, movement patterns and tissue deformation. Another potential approach is to generate mutant animals exhibiting altered movement patterns. Finally, to increase the resolution of biological outcome assessment of different mechanical signals, it is necessary to improve methods for data analysis, including single-cell transcriptome analysis and single-molecule fluorescent in situ hybridization. Overall, these combined efforts will hopefully allow us to move towards a more mechanistic understanding of the forces that operate in the context of musculoskeletal system development and disease.
Acknowledgements
We thank Nitzan Konstantin for expert editorial assistance; Dr Ronen Schweizer from the Shriners Hospital for Children, Portland, Oregon, USA and Dr Peleg Hasson from the Technion-Israel Institute of Technology, Haifa, Israel for critical reading and suggestions; and special thanks to all members of the E.Z. laboratory for encouragement and advice.
Funding
This Review was supported by grants from the European Research Council (ERC) (grant no. 310098) and the following internal Weizmann Institute internal funds: the Jeanne and Joseph Nissim Foundation for Life Sciences Research, the Y. Leon Benoziyo Institute for Molecular Medicine, Beth Rom-Rymer, the Estate of David Levinson, the Jaffe Bernard and Audrey Foundation, Georges Lustgarten Cancer Research Fund, the David and Fela Shapell Family Center for Genetic Disorders, the David and Fela Shapell Family Foundation INCPM Fund for Preclinical Studies, and the Estate of Bernard Bishin for the WIS-Clalit Program.
Footnotes
Competing interests
The authors declare no competing or financial interests.
References
- Barik A., Zhang B., Sohal G. S., Xiong W.-C. and Mei L. (2014). Crosstalk between Agrin and Wnt signaling pathways in development of vertebrate neuromuscular junction. Dev. Neurobiol. 74, 828-838. 10.1002/dneu.22190 [DOI] [PubMed] [Google Scholar]
- Bastow E. R., Lamb K. J., Lewthwaite J. C., Osborne A. C., Kavanagh E., Wheeler-Jones C. P. D. and Pitsillides A. A. (2005). Selective activation of the MEK-ERK pathway is regulated by mechanical stimuli in forming joints and promotes pericellular matrix formation. J. Biol. Chem. 280, 11749-11758. 10.1074/jbc.M414495200 [DOI] [PubMed] [Google Scholar]
- Bekoff A. (1981). Embryonic development of chick motor behaviour. Trends Neurosci. 4, 181-184. 10.1016/0166-2236(81)90059-X [DOI] [Google Scholar]
- Benjamin M., Toumi H., Ralphs J. R., Bydder G., Best T. M. and Milz S. (2006). Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J. Anat. 208, 471-490. 10.1111/j.1469-7580.2006.00540.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Davey D. F. and Marshall J. J. (1983). The growth of nerves in relation to the formation of premuscle cell masses in the developing chick forelimb. J. Comp. Neurol. 215, 217-227. 10.1002/cne.902150209 [DOI] [PubMed] [Google Scholar]
- Berendsen A. D. and Olsen B. R. (2015). Bone development. Bone 80, 14-18. 10.1016/j.bone.2015.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi W., Deng J. M., Zhang Z., Behringer R. R. and de Crombrugghe B. (1999). Sox9 is required for cartilage formation. Nat. Genet. 22, 85-89. 10.1038/8792 [DOI] [PubMed] [Google Scholar]
- Birch H. L., Thorpe C. T. and Rumian A. P. (2013). Specialisation of extracellular matrix for function in tendons and ligaments. Muscles Ligaments Tendons J. 3, 12-22. 10.11138/mltj/2013.3.1.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biressi S., Molinaro M. and Cossu G. (2007). Cellular heterogeneity during vertebrate skeletal muscle development. Dev. Biol. 308, 281-293. 10.1016/j.ydbio.2007.06.006 [DOI] [PubMed] [Google Scholar]
- Blecher R., Krief S., Galili T., Biton I. E., Stern T., Assaraf E., Levanon D., Appel E., Anekstein Y., Agar G. et al. (2017a). The proprioceptive system masterminds spinal alignment: insight into the mechanism of scoliosis. Dev. Cell 42, 388-399.e3. 10.1016/j.devcel.2017.07.022 [DOI] [PubMed] [Google Scholar]
- Blecher R., Krief S., Galili T., Assaraf E., Stern T., Anekstein Y., Agar G. and Zelzer E. (2017b). The proprioceptive system regulates morphologic restoration of fractured bones. Cell Rep. 20, 1775-1783. 10.1016/j.celrep.2017.07.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz E., Viukov S., Sharir A., Shwartz Y., Galloway J. L., Pryce B. A., Johnson R. L., Tabin C. J., Schweitzer R. and Zelzer E. (2009). Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev. Cell 17, 861-873. 10.1016/j.devcel.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz E., Sharir A., Akiyama H. and Zelzer E. (2013). Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development 140, 2680-2690. 10.1242/dev.093906 [DOI] [PubMed] [Google Scholar]
- Breidenbach A. P., Aschbacher-Smith L., Lu Y., Dyment N. A., Liu C.-F., Liu H., Wylie C., Rao M., Shearn J. T., Rowe D. W. et al. (2015). Ablating Hedgehog signaling in tenocytes during development impairs biomechanics and matrix organization of the adult murine patellar tendon enthesis. J. Orthop. Res. 33, 1142-1151. 10.1002/jor.22899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadus A. E., Macica C. and Chen X. (2007). The PTHrP functional domain is at the gates of endochondral bones. Ann. N. Y. Acad. Sci. 1116, 65-81. 10.1196/annals.1402.061 [DOI] [PubMed] [Google Scholar]
- Brunt L. H., Norton J. L., Bright J. A., Rayfield E. J. and Hammond C. L. (2015). Finite element modelling predicts changes in joint shape and cell behaviour due to loss of muscle strain in jaw development. J. Biomech. 48, 3112-3122. 10.1016/j.jbiomech.2015.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt L. H., Skinner R. E. H., Roddy K. A., Araujo N. M., Ray E. J. and Hammond C. L. (2016). Differential effects of altered patterns of movement and strain on joint cell behaviour and skeletal morphogenesis. Osteoarthritis Cartilage 24, 1940-1950. 10.1016/j.joca.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt L. H., Begg K., Kague E., Cross S. and Hammond C. L. (2017). Wnt signalling controls the response to mechanical loading during zebrafish joint development. Development 144, 2798-2809. 10.1242/dev.153528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M., Bajard L., Chang T., Daubas P., Hadchouel J., Meilhac S., Montarras D., Rocancourt D. and Relaix F. (2003). The formation of skeletal muscle: from somite to limb. J. Anat. 202, 59-68. 10.1046/j.1469-7580.2003.00139.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carry M. R., Morita M. and Nornes H. O. (1983). Morphogenesis of motor endplates along the proximodistal axis of the mouse hindlimb. Anat. Rec. 207, 473-485. 10.1002/ar.1092070309 [DOI] [PubMed] [Google Scholar]
- Chal J. and Pourquié O. (2017). Making muscle: skeletal myogenesis in vivo and in vitro. Development 144, 2104-2122. 10.1242/dev.151035 [DOI] [PubMed] [Google Scholar]
- Charvet B., Guiraud A., Malbouyres M., Zwolanek D., Guillon E., Bretaud S., Monnot C., Schulze J., Bader H. L., Allard B. et al. (2013). Knockdown of col22a1 gene in zebrafish induces a muscular dystrophy by disruption of the myotendinous junction. Development 140, 4602-4613. 10.1242/dev.096024 [DOI] [PubMed] [Google Scholar]
- Chen J. W. and Galloway J. L. (2014). The development of zebrafish tendon and ligament progenitors. Development 141, 2035-2045. 10.1242/dev.104067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.-H., Hippenmeyer S., Arber S. and Frank E. (2003). Development of the monosynaptic stretch reflex circuit. Curr. Opin. Neurobiol. 13, 96-102. 10.1016/S0959-4388(03)00006-0 [DOI] [PubMed] [Google Scholar]
- Chen X., Macica C., Nasiri A., Judex S. and Broadus A. E. (2007). Mechanical regulation of PTHrP expression in entheses. Bone 41, 752-759. 10.1016/j.bone.2007.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler A. T., Szczot M., Bharucha-Goebel D., Čeko M., Donkervoort S., Laubacher C., Hayes L. H., Alter K., Zampieri C., Stanley C. et al. (2016). The role of PIEZO2 in human mechanosensation. N. Engl. J. Med. 375, 1355-1364. 10.1056/NEJMoa1602812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard-Pelletier G., Jahnsen E. D. and Jones E. A. V. (2013). Increased shear stress inhibits angiogenesis in veins and not arteries during vascular development. Angiogenesis 16, 71-83. 10.1007/s10456-012-9300-2 [DOI] [PubMed] [Google Scholar]
- Colasanto M. P., Eyal S., Mohassel P., Bamshad M., Bonnemann C. G., Zelzer E., Moon A. M. and Kardon G. (2016). Development of a subset of forelimb muscles and their attachment sites requires the ulnar-mammary syndrome gene Tbx3. Dis. Model. Mech. 9, 1257-1269. 10.1242/dmm.025874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai G. and Tajbakhsh S. (2014). Molecular and cellular regulation of skeletal myogenesis. Curr. Top. Dev. Biol. 110, 1-73. 10.1016/B978-0-12-405943-6.00001-4 [DOI] [PubMed] [Google Scholar]
- Cooper L. N., Dawson S. D., Reidenberg J. S. and Berta A. (2007). Neuromuscular anatomy and evolution of the cetacean forelimb. Anat. Rec. 290, 1121-1137. 10.1002/ar.20571 [DOI] [PubMed] [Google Scholar]
- Coste B., Houge G., Murray M. F., Stitziel N., Bandell M., Giovanni M. A., Philippakis A., Hoischen A., Riemer G., Steen U. et al. (2013). Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of distal arthrogryposis. Proc. Natl. Acad. Sci. USA 110, 4667-4672. 10.1073/pnas.1221400110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabid H., Perez-Gonzalez A. P. and Robitaille R. (2014). Neuromuscular synaptogenesis: coordinating partners with multiple functions. Nat. Rev. Neurosci. 15, 703-718. 10.1038/nrn3821 [DOI] [PubMed] [Google Scholar]
- de Lima J. E., Bonnin M. A., Birchmeier C. and Duprez D. (2016). Muscle contraction is required to maintain the pool of muscle progenitors via yap and notch during fetal myogenesis. Elife 5, e15593 10.7554/eLife.15593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lisio M., Jensen T., Sukiennik R. A., Huntsman H. D. and Boppart M. D. (2014). Substrate and strain alter the muscle-derived mesenchymal stem cell secretome to promote myogenesis. Stem Cell Res. Ther. 5, 74 10.1186/scrt463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries J. I. P., Visser G. H. A. and Prechtl H. F. R. (1982). The emergence of fetal behaviour. I. Qualitative aspects. Early Hum. Dev. 7, 301-322. 10.1016/0378-3782(82)90033-0 [DOI] [PubMed] [Google Scholar]
- Decker R. S., Koyama E. and Pacifici M. (2014). Genesis and morphogenesis of limb synovial joints and articular cartilage. Matrix Biol. 39, 5-10. 10.1016/j.matbio.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Wu A., Li P., Li G., Qin L., Song H. and Mak K. K. (2016). Yap1 regulates multiple steps of chondrocyte differentiation during skeletal development and bone repair. Cell Rep. 14, 2224-2237. 10.1016/j.celrep.2016.02.021 [DOI] [PubMed] [Google Scholar]
- Desprat N., Supatto W., Pouille P.-A., Beaurepaire E. and Farge E. (2008). Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev. Cell 15, 470-477. 10.1016/j.devcel.2008.07.009 [DOI] [PubMed] [Google Scholar]
- Drachman D. B. and Sokoloff L. (1966). The role of movement in embryonic joint development. Dev. Biol. 14, 401-420. 10.1016/0012-1606(66)90022-4 [DOI] [Google Scholar]
- Dyment N. A., Breidenbach A. P., Schwartz A. G., Russell R. P., Aschbacher-Smith L., Liu H., Hagiwara Y., Jiang R., Thomopoulos S., Butler D. L. et al. (2015). Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Dev. Biol. 405, 96-107. 10.1016/j.ydbio.2015.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edom-Vovard F., Schuler B., Bonnin M.-A., Teillet M.-A. and Duprez D. (2002). Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev. Biol. 247, 351-366. 10.1006/dbio.2002.0707 [DOI] [PubMed] [Google Scholar]
- Eyal S., Blitz E., Shwartz Y., Akiyama H., Schweitzer R. and Zelzer E. (2015). On the development of the patella. Development 142, 1831-1839. 10.1242/dev.121970 [DOI] [PubMed] [Google Scholar]
- Fahim M. A. (1989). Rapid neuromuscular remodeling following limb immobilization. Anat. Rec. 224, 102-109. 10.1002/ar.1092240113 [DOI] [PubMed] [Google Scholar]
- Fell H. B. and Canti R. G. (1934). Experiments on the development in vitro of the avian knee-joint. Proc. R. Soc. B1176, 316-351. 10.1098/rspb.1934.0076 [DOI] [Google Scholar]
- Florez-Paz D., Bali K. K., Kuner R. and Gomis A. (2016). A critical role for Piezo2 channels in the mechanotransduction of mouse proprioceptive neurons. Sci. Rep. 6, 25923 10.1038/srep25923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford C. A., Nowlan N. C., Thomopoulos S. and Killian M. L. (2017). Effects of imbalanced muscle loading on hip joint development and maturation. J. Orthop. Res. 35, 1128-1136. 10.1002/jor.23361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco Botelho J., Smith-Paredes D., Soto-Acuña S., Mpodozis J., Palma V. and Vargas A. O. (2015). Skeletal plasticity in response to embryonic muscular activity underlies the development and evolution of the perching digit of birds. Sci. Rep. 5, 9840 10.1038/srep09840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galie P. A., Nguyen D.-H. T., Choi C. K., Cohen D. M., Janmey P. A. and Chen C. S. (2014). Fluid shear stress threshold regulates angiogenic sprouting. Proc. Natl. Acad. Sci. USA 111, 7968-7973. 10.1073/pnas.1310842111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaut L. and Duprez D. (2016). Tendon development and diseases. Wiley Interdiscip. Rev. Dev. Biol. 5, 5-23. 10.1002/wdev.201 [DOI] [PubMed] [Google Scholar]
- Gaut L., Robert N., Delalande A., Bonnin M.-A., Pichon C. and Duprez D. (2016). EGR1 regulates transcription downstream of mechanical signals during tendon formation and healing. PLoS ONE 11, e0166237 10.1371/journal.pone.0166237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautel M. and Djinovic-Carugo K. (2016). The sarcomeric cytoskeleton: from molecules to motion. J. Exp. Biol. 219, 135-145. 10.1242/jeb.124941 [DOI] [PubMed] [Google Scholar]
- Germiller J. A. and Goldstein S. A. (1997). Structure and function of embryonic growth plate in the absence of functioning skeletal muscle. J. Orthop. Res. 15, 362-370. 10.1002/jor.1100150308 [DOI] [PubMed] [Google Scholar]
- Giorgi M., Carriero A., Shefelbine S. J. and Nowlan N. C. (2015). Effects of normal and abnormal loading conditions on morphogenesis of the prenatal hip joint: application to hip dysplasia. J. Biomech. 48, 3390-3397. 10.1016/j.jbiomech.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez C., David V., Peet N. M., Vico L., Chenu C., Malaval L. and Skerry T. M. (2007). Absence of mechanical loading in utero influences bone mass and architecture but not innervation in Myod-Myf5-deficient mice. J. Anat. 210, 259-271. 10.1111/j.1469-7580.2007.00698.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon N. (1998). Arthrogryposis multiplex congenita. Brain Dev. 20, 507-511. 10.1016/S0387-7604(98)00037-0 [DOI] [PubMed] [Google Scholar]
- Gordon L. R., Gribble K. D., Syrett C. M. and Granato M. (2012). Initiation of synapse formation by Wnt-induced MuSK endocytosis. Development 139, 1023-1033. 10.1242/dev.071555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R. (1975). The functional role of the muscle spindles-facts and hypotheses. Brain 98, 531-556. 10.1093/brain/98.4.531 [DOI] [PubMed] [Google Scholar]
- Guerquin M.-J., Charvet B., Nourissat G., Havis E., Ronsin O., Bonnin M.-A., Ruggiu M., Olivera-Martinez I., Robert N., Lu Y. et al. (2013). Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J. Clin. Invest. 123, 3564-3576. 10.1172/JCI67521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Day T. F., Jiang X., Garrett-Beal L., Topol L. and Yang Y. (2004). Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 18, 2404-2417. 10.1101/gad.1230704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. G. (1985a). In utero movement and use of limbs are necessary for normal growth: a study of individuals with arthrogryposis. Prog. Clin. Biol. Res. 200, 155-162. [PubMed] [Google Scholar]
- Hall J. G. (1985b). Genetic aspects of arthrogryposis. Clin. Orthop. Relat. Res. 194, 44-53. [PubMed] [Google Scholar]
- Hall B. K. and Herring S. W. (1990). Paralysis and growth of the musculoskeletal system in the embryonic chick. J. Morphol. 206, 45-56. 10.1002/jmor.1052060105 [DOI] [PubMed] [Google Scholar]
- Hall J. G., Opitz J. M. and Reynolds J. F. (1986). Analysis of Pena Shokeir phenotype. Am. J. Med. Genet. 25, 99-117. 10.1002/ajmg.1320250112 [DOI] [PubMed] [Google Scholar]
- Hamburger V. and Balaban M. (1963). Observations and experiments on spontaneous rhythmical behavior in the chick embryo. Dev. Biol. 6, 533-545. 10.1016/0012-1606(63)90140-4 [DOI] [PubMed] [Google Scholar]
- Hamburger V. and Waugh M. (1940). The primary development of the skeleton in nerveless and poorly innervated limb transplants of chick embryos. Physiol. Zool. 13, 367-382. 10.1086/physzool.13.4.30151585 [DOI] [Google Scholar]
- Harris A. J., Duxson M. J., Fitzsimons R. B. and Rieger F. (1989). Myonuclear birthdates distinguish the origins of primary and secondary myotubes in embryonic mammalian skeletal muscles. Development 107, 771-784. [DOI] [PubMed] [Google Scholar]
- Hartmann C. and Tabin C. J. (2001). Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell 104, 341-351. 10.1016/S0092-8674(01)00222-7 [DOI] [PubMed] [Google Scholar]
- Hasson P., DeLaurier A., Bennett M., Grigorieva E., Naiche L. A., Papaioannou V. E., Mohun T. J. and Logan M. P. O. (2010). Tbx4 and tbx5 acting in connective tissue are required for limb muscle and tendon patterning. Dev. Cell 18, 148-156. 10.1016/j.devcel.2009.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson P., Volk T. and Salzberg A. (2017). Building functional units of movement-generation and movement-sensation in the embryo. Int. J. Dev. Biol. 61, 171-178. 10.1387/ijdb.160279as [DOI] [PubMed] [Google Scholar]
- Havis E., Bonnin M.-A., Olivera-Martinez I., Nazaret N., Ruggiu M., Weibel J., Durand C., Guerquin M.-J., Bonod-Bidaud C., Ruggiero F. et al. (2014). Transcriptomic analysis of mouse limb tendon cells during development. Development 141, 3683-3696. 10.1242/dev.108654 [DOI] [PubMed] [Google Scholar]
- Havis E., Bonnin M.-A., Esteves de Lima J., Charvet B., Milet C. and Duprez D. (2016). TGFβ and FGF promote tendon progenitor fate and act downstream of muscle contraction to regulate tendon differentiation during chick limb development. Development 143, 3839-3851. 10.1242/dev.136242 [DOI] [PubMed] [Google Scholar]
- Heegaard J. H., Beaupré G. S. and Carter D. R. (1999). Mechanically modulated cartilage growth may regulate joint surface morphogenesis. J. Orthop. Res. 17, 509-517. 10.1002/jor.1100170408 [DOI] [PubMed] [Google Scholar]
- Heer N. C. and Martin A. C. (2017). Tension, contraction and tissue morphogenesis. Development 144, 4249-4260. 10.1242/dev.151282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbest C. (1901). Formative Reize in der tierischen Ontogenese. Ein Beitrag zum Verständnis der tierischen Embryonalentwicklung. Leipzig: Georgi. [Google Scholar]
- Hosseini A. and Hogg D. A. (1991). The effects of paralysis on skeletal development in the chick embryo. I. General effects. J. Anat. 177, 159-168. [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Riordan T. J., Wang L., Eyal S., Zelzer E., Brigande J. V. and Schweitzer R. (2013). Repositioning forelimb superficialis muscles: tendon attachment and muscle activity enable active relocation of functional myofibers. Dev. Cell 26, 544-551. 10.1016/j.devcel.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Lu H. H. and Schweitzer R. (2015). Molecular regulation of tendon cell fate during development. J. Orthop. Res. 33, 800-812. 10.1002/jor.22834 [DOI] [PubMed] [Google Scholar]
- Ishijima M., Tsuji K., Rittling S. R., Yamashita T., Kurosawa H., Denhardt D. T., Nifuji A. and Noda M. (2002). Resistance to unloading-induced three-dimensional bone loss in osteopontin-deficient mice. J. Bone Miner. Res. 17, 661-667. 10.1359/jbmr.2002.17.4.661 [DOI] [PubMed] [Google Scholar]
- Ito Y., Toriuchi N., Yoshitaka T., Ueno-Kudoh H., Sato T., Yokoyama S., Nishida K., Akimoto T., Takahashi M., Miyaki S. et al. (2010). The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc. Natl. Acad. Sci. USA 107, 10538-10542. 10.1073/pnas.1000525107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan E., Matsumoto A., Rafiq A. M., Hashimoto R., Inoue T., Udagawa J., Sekine J. and Otani H. (2014). Fetal jaw movement affects Ihh signaling in mandibular condylar cartilage development: the possible role of Ihh as mechanotransduction mediator. Arch. Oral Biol. 59, 1108-1118. 10.1016/j.archoralbio.2014.06.009 [DOI] [PubMed] [Google Scholar]
- Jaramillo F., Vicini S. and Schuetze S. M. (1988). Embryonic acetylcholine receptors guarantee spontaneous contractions in rat developing muscle. Nature 335, 66-68. 10.1038/335066a0 [DOI] [PubMed] [Google Scholar]
- Jesudason E. C., Smith N. P., Connell M. G., Spiller D. G., White M. R. H., Fernig D. G. and Losty P. D. (2006). Peristalsis of airway smooth muscle is developmentally regulated and uncoupled from hypoplastic lung growth. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L559-L565. 10.1152/ajplung.00498.2005 [DOI] [PubMed] [Google Scholar]
- Kahn J., Shwartz Y., Blitz E., Krief S., Sharir A., Breitel D. A., Rattenbach R., Relaix F., Maire P., Rountree R. B. et al. (2009). Muscle contraction is necessary to maintain joint progenitor cell fate. Dev. Cell 16, 734-743. 10.1016/j.devcel.2009.04.013 [DOI] [PubMed] [Google Scholar]
- Kardon G. (1998). Muscle and tendon morphogenesis in the avian hind limb. Development 125, 4019-4032. [DOI] [PubMed] [Google Scholar]
- Kardon G., Harfe B. D. and Tabin C. J. (2003). A Tcf4-positive mesodermal population provides a prepattern for vertebrate limb muscle patterning. Dev. Cell 5, 937-944. 10.1016/S1534-5807(03)00360-5 [DOI] [PubMed] [Google Scholar]
- Kavanagh E., Church V. L., Osborne A. C., Lamb K. J., Archer C. W., Francis-West P. H. and Pitsillides A. A. (2006). Differential regulation of GDF-5 and FGF-2/4 by immobilisation in ovo exposes distinct roles in joint formation. Dev. Dyn. 235, 826-834. 10.1002/dvdy.20679 [DOI] [PubMed] [Google Scholar]
- Kayama T., Mori M., Ito Y., Matsushima T., Nakamichi R., Suzuki H., Ichinose S., Saito M., Marumo K. and Asahara H. (2016). Gtf2ird1-dependent mohawk expression regulates mechanosensing properties of the tendon. Mol. Cell. Biol. 36, 1297-1309. 10.1128/MCB.00950-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkorogiannis T. (2004). Somatic and intramuscular distribution of muscle spindles and their relation to muscular angiotypes. J. Theor. Biol. 229, 263-280. 10.1016/j.jtbi.2004.03.019 [DOI] [PubMed] [Google Scholar]
- Kronenberg H. M. (2003). Developmental regulation of the growth plate. Nature 423, 332-336. 10.1038/nature01657 [DOI] [PubMed] [Google Scholar]
- Kutys M. L. and Chen C. S. (2016). Forces and mechanotransduction in 3D vascular biology. Curr. Opin. Cell Biol. 42, 73-79. 10.1016/j.ceb.2016.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis W. J. and Silver F. H. (2002). The structure and function of normally mineralizing avian tendons. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 133, 1135-1157. 10.1016/S1095-6433(02)00248-9 [DOI] [PubMed] [Google Scholar]
- Lee W., Leddy H. A., Chen Y., Hee S., Zelenski N. A., Mcnulty A. L., Wu J., Beicker K. N., Coles J., Zauscher S. et al. (2014). Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc. Natl. Acad. Sci. USA 111, E5114-E5122. 10.1073/pnas.1414298111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legay C. and Mei L. (2017). Moving forward with the neuromuscular junction. J. Neurochem. 142, 59-63. 10.1111/jnc.14028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejard V., Blais F., Guerquin M.-J., Bonnet A., Bonnin M.-A., Havis E., Malbouyres M., Bidaud C. B., Maro G., Gilardi-Hebenstreit P. et al. (2011). EGR1 and EGR2 involvement in vertebrate tendon differentiation. J. Biol. Chem. 286, 5855-5867. 10.1074/jbc.M110.153106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelkes G. (1958). Experiments in vitro on the role of movement in the development of joints. J. Embryol. Exp. Morphol. 6, 183-186. [PubMed] [Google Scholar]
- Lemke S. B. and Schnorrer F. (2017). Mechanical forces during muscle development. Mech. Dev. 144, 92-101. 10.1016/j.mod.2016.11.003 [DOI] [PubMed] [Google Scholar]
- Lin W., Burgess R. W., Dominguez B., Pfaff S. L., Sanes J. R., Lee K.-F., Burgess R. W., Dominguez B., Pfaff S. L., Sanes J. R. et al. (2001). Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature 410, 1057-1064. 10.1038/35074025 [DOI] [PubMed] [Google Scholar]
- Liu W., Watson S. S., Lan Y., Keene D. R., Ovitt C. E., Liu H., Schweitzer R. and Jiang R. (2010). The atypical homeodomain transcription factor Mohawk controls tendon morphogenesis. Mol. Cell. Biol. 30, 4797-4807. 10.1128/MCB.00207-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.-F., Breidenbach A., Aschbacher-Smith L., Butler D. and Wylie C. (2013). A role for Hedgehog signaling in the differentiation of the insertion site of the patellar tendon in the mouse. PLoS ONE 8, e65411 10.1371/journal.pone.0065411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Schwartz A. G., Birman V., Thomopoulos S. and Genin G. M. (2014). Stress amplification during development of the tendon-to-bone attachment. Biomech. Model. Mechanobiol. 13, 973-983. 10.1007/s10237-013-0548-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lømo T., Massoulié J. and Vigny M. (1985). Stimulation of denervated rat soleus muscle with fast and slow activity patterns induces different expression of acetylcholinesterase molecular forms. J. Neurosci. 5, 1180-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorda-Diez C. I., Montero J. A., Martinez-Cue C., Garcia-Porrero J. A. and Hurle J. M. (2009). Transforming growth factors beta coordinate cartilage and tendon differentiation in the developing limb mesenchyme. J. Biol. Chem. 284, 29988-29996. 10.1074/jbc.M109.014811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucitti J. L., Jones E. A. V., Huang C., Chen J., Fraser S. E. and Dickinson M. E. (2007). Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 134, 3317-3326. 10.1242/dev.02883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Sakabe T., Sunaga A., Sakai K., Rivera A. L., Keene D. R., Sasaki T., Stavnezer E., Iannotti J., Schweitzer R. et al. (2011). Conversion of mechanical force into TGF-β-mediated biochemical signals. Curr. Biol. 21, 933-941. 10.1016/j.cub.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda E., Ye S., Wang W., Bader D. L., Knight M. M. and Lee D. A. (2012). Gap junction permeability between tenocytes within tendon fascicles is suppressed by tensile loading. Biomech. Model. Mechanobiol. 11, 439-447. 10.1007/s10237-011-0323-1 [DOI] [PubMed] [Google Scholar]
- Maier A. (1997). Development and regeneration of muscle spindles in mammals and birds. Int. J. Dev. Biol. 41, 1-17. [PubMed] [Google Scholar]
- Mammoto T. and Ingber D. E. (2010). Mechanical control of tissue and organ development. Development 137, 1407-1420. 10.1242/dev.024166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto T., Mammoto A. and Ingber D. E. (2013). Mechanobiology and developmental control. Annu. Rev. Cell Dev. Biol. 29, 27-61. 10.1146/annurev-cellbio-101512-122340 [DOI] [PubMed] [Google Scholar]
- Martin P. (1990). Tissue patterning in the developing mouse limb. Int. J. Dev. Biol. 34, 323-336. [PubMed] [Google Scholar]
- Mathew S. J., Hansen J. M., Merrell A. J., Murphy M. M., Lawson J. A., Hutcheson D. A., Hansen M. S., Angus-Hill M. and Kardon G. (2011). Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development 138, 371-384. 10.1242/dev.057463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillin M. J., Beck A. E., Chong J. X., Shively K. M., Buckingham K. J., Gildersleeve H. I. S., Aracena M. I., Aylsworth A. S., Bitoun P., Carey J. C. et al. (2014). Mutations in PIEZO2 cause Gordon syndrome, Marden-Walker syndrome, and distal arthrogryposis type 5. Am. J. Hum. Genet. 94, 734-744. 10.1016/j.ajhg.2014.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messéant J., Ezan J., Delers P., Glebov K., Marchiol C., Lager F., Renault G., Tissir F., Montcouquiol M., Sans N. et al. (2017). Wnt proteins contribute to neuromuscular junction formation through distinct signaling pathways. Development 144, 1712-1724. 10.1242/dev.146167 [DOI] [PubMed] [Google Scholar]
- Mikic B., Johnson T. L., Chhabra A. B., Schalet B. J., Wong M. and Hunziker E. B. (2000a). Differential effects of embryonic immobilization on the development of fibrocartilaginous skeletal elements. J. Rehabil. Res. Dev. 37, 127-133. [PubMed] [Google Scholar]
- Mikic B., Wong M., Chiquet M. and Hunziker E. B. (2000b). Mechanical modulation of tenascin-C and collagen-XII expression during avian synovial joint formation. J. Orthop. Res. 18, 406-415. 10.1002/jor.1100180312 [DOI] [PubMed] [Google Scholar]
- Mikic B., Isenstein A. L. and Chhabra A. (2004). Mechanical modulation of cartilage structure and function during embryogenesis in the chick. Ann. Biomed. Eng. 32, 18-25. 10.1023/B:ABME.0000007787.39262.a7 [DOI] [PubMed] [Google Scholar]
- Mitrovic D. R. (1977). Development of the metatarsophalangeal joint of the chick embryo: morphological, ultrastructural and histochemical studies. Am. J. Anat. 150, 333-347. 10.1002/aja.1001500207 [DOI] [PubMed] [Google Scholar]
- Mitrovic D. (1982). Development of the articular cavity in paralyzed chick embryos and in chick embryo limb buds cultured on chorioallantoic membranes. Acta Anat. 113, 313-324. 10.1159/000145566 [DOI] [PubMed] [Google Scholar]
- Moore J. C. (1984). The Golgi tendon organ: a review and update. Am. J. Occup. Ther. 38, 227-236. 10.5014/ajot.38.4.227 [DOI] [PubMed] [Google Scholar]
- Murphy M. and Kardon G. (2011). Origin of vertebrate limb muscle: the role of progenitor and myoblast populations. Myogenesis 96, 1-32. 10.1016/B978-0-12-385940-2.00001-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. D. and Drachman D. B. (1969). The role of movement in the development of joints and related structures: the head and neck in the chick embryo. J. Embryol. Exp. Morphol. 22, 349-371. [PubMed] [Google Scholar]
- Nelson C. M. and Gleghorn J. P. (2012). Sculpting organs: mechanical regulation of tissue development. Annu. Rev. Biomed. Eng. 14, 129-154. 10.1146/annurev-bioeng-071811-150043 [DOI] [PubMed] [Google Scholar]
- Nowlan N. C., Murphy P. and Prendergast P. J. (2008). A dynamic pattern of mechanical stimulation promotes ossification in avian embryonic long bones. J. Biomech. 41, 249-258. 10.1016/j.jbiomech.2007.09.031 [DOI] [PubMed] [Google Scholar]
- Nowlan N. C., Bourdon C., Dumas G., Tajbakhsh S., Prendergast P. J. and Murphy P. (2010). Developing bones are differentially affected by compromised skeletal muscle formation. Bone 46, 1275-1285. 10.1016/j.bone.2009.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowlan N. C., Chandaria V. and Sharpe J. (2014). Immobilized chicks as a model system for early-onset developmental dysplasia of the hip. J. Orthop. Res. 32, 777-785. 10.1002/jor.22606 [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Houenou L., Pincon-raymond M., Powell J. A., Rieger F. and Standish J. (1986). The development of motoneurons in the embryonic spinal cord of the mouse mutant, muscular dysgenesis (mdg/mdg): survival, morphology, and biochemical differentiation. Dev. Biol. 436, 426-436. 10.1016/0012-1606(86)90207-1 [DOI] [PubMed] [Google Scholar]
- Osborne A. C., Lamb K. J., Lewthwaite J. C., Dowthwaite G. P. and Pitsillides A. A. (2002). Short-term rigid and flaccid paralyses diminish growth of embryonic chick limbs and abrogate joint cavity formation but differentially preserve pre-cavitated joints. J. Musculoskelet. Neuronal Interact. 2, 448-456. [PubMed] [Google Scholar]
- Pa A. C. and Pai A. C. (1965). Developmental genetics of a lethal mutation, muscular dysgenesis (Mdg), in the mouse. II. Developmental analysis. Dev. Biol. 11, 93-109. 10.1016/0012-1606(65)90039-4 [DOI] [PubMed] [Google Scholar]
- Pai A. C. (1965). Developmental genetics of a lethal mutation, muscular dysgenesis (Mdg), in the mouse. I. Genetic analysis and gross morphology. Dev. Biol. 11, 82-92. 10.1016/0012-1606(65)90038-2 [DOI] [PubMed] [Google Scholar]
- Parpaite T. and Coste B. (2017). Piezo channels. Curr. Biol. 27, R250-R252. 10.1016/j.cub.2017.01.048 [DOI] [PubMed] [Google Scholar]
- Persson M. (1983). The role of movements in the development of sutural and diarthrodial joints tested by long-term paralysis of chick embryos. J. Anat. 137, 591-599. [PMC free article] [PubMed] [Google Scholar]
- Pinçon-Raymond M. and Rieger F. (1982). Extensive multiple innervation and abnormal synaptogenesis in muscular dysgenesis (mdg/mdg) in the mouse embryo. Reprod. Nutr. Dev. 22, 217-226. 10.1051/rnd:19820208 [DOI] [PubMed] [Google Scholar]
- Pittman R. and Oppenheim R. W. (1979). Cell death of motoneurons in the chick embryo spinal cord. IV. Evidence that a functional neuromuscular interaction is involved in the regulation of naturally occurring cell death and the stabilization of synapses. J. Comp. Neurol. 187, 425-446. 10.1002/cne.901870210 [DOI] [PubMed] [Google Scholar]
- Pollard A. S., Charlton B. G., Hutchinson J. R., Gustafsson T., Mcgonnell I. M., Timmons J. A. and Pitsillides A. A. (2017). Limb proportions show developmental plasticity in response to embryo movement. Sci. Rep. 7, 1-16. 10.1038/srep41926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov C., Burggraf M., Kreja L., Ignatius A., Schieker M. and Docheva D. (2015). Mechanical stimulation of human tendon stem/progenitor cells results in upregulation of matrix proteins, integrins and MMPs, and activation of p38 and ERK1/2 kinases. BMC Mol. Biol. 16, 6 10.1186/s12867-015-0036-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce B. A., Watson S. S., Murchison N. D., Staverosky J. A., Dünker N. and Schweitzer R. (2009). Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development 136, 1351-1361. 10.1242/dev.027342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rais Y., Reich A., Simsa-Maziel S., Moshe M., Idelevich A., Kfir T., Miosge N. and Monsonego-Ornan E. (2015). The growth plate's response to load is partially mediated by mechano-sensing via the chondrocytic primary cilium. Cell. Mol. Life Sci. 72, 597-615. 10.1007/s00018-014-1690-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddy K. A., Prendergast P. J. and Murphy P. (2011a). Mechanical influences on morphogenesis of the knee joint revealed through morphological, molecular and computational analysis of immobilised embryos. PLoS ONE 6, e17526 10.1371/journal.pone.0017526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddy K. A., Kelly G. M., van Es M. H., Murphy P. and Prendergast P. J. (2011b). Dynamic patterns of mechanical stimulation co-localise with growth and cell proliferation during morphogenesis in the avian embryonic knee joint. J. Biomech. 44, 143-149. 10.1016/j.jbiomech.2010.08.039 [DOI] [PubMed] [Google Scholar]
- Rodríguez J. I., Palacios J., Ruiz A., Sanchez M., Alvarez I. and Demiguel E. (1992). Morphological changes in long bone development in fetal akinesia deformation sequence: an experimental study in curarized rat fetuses. Teratology 45, 213-221. 10.1002/tera.1420450215 [DOI] [PubMed] [Google Scholar]
- Rolfe R. A., Nowlan N. C., Kenny E. M., Cormican P., Morris D. W., Prendergast P. J., Kelly D. and Murphy P. (2014). Identification of mechanosensitive genes during skeletal development: alteration of genes associated with cytoskeletal rearrangement and cell signalling pathways. BMC Genomics 15, 48 10.1186/1471-2164-15-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe R. A., Bezer J. H., Kim T., Zaidon A. Z., Oyen M. L., Iatridis J. C. and Nowlan N. C. (2017). Abnormal fetal muscle forces result in defects in spinal curvature and alterations in vertebral segmentation and shape. J. Orthop. Res. 35, 2135-2144. 10.1002/jor.23518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romereim S. M., Conoan N. H., Chen B. and Dudley A. T. (2014). A dynamic cell adhesion surface regulates tissue architecture in growth plate cartilage. Development 141, 2085-2095. 10.1242/dev.105452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rot C., Stern T., Blecher R., Friesem B. and Zelzer E. (2014). A mechanical jack-like mechanism drives spontaneous fracture healing in neonatal mice. Dev. Cell 31, 159-170. 10.1016/j.devcel.2014.08.026 [DOI] [PubMed] [Google Scholar]
- Rot-Nikcevic I., Reddy T., Downing K. J., Belliveau A. C., Hallgrímsson B., Hall B. K. and Kablar B. (2006). Myf5−/−: MyoD−/− amyogenic fetuses reveal the importance of early contraction and static loading by striated muscle in mouse skeletogenesis. Dev. Genes Evol. 216, 1-9. 10.1007/s00427-005-0024-9 [DOI] [PubMed] [Google Scholar]
- Ruano-Gil D., Nardi-Vilardaga J. and Tejedo-Mateu A. (1978). Influence of extrinsic factors on the development of the articular system. Acta Anat. 101, 36-44. 10.1159/000144947 [DOI] [PubMed] [Google Scholar]
- Ruano-Gil D., Nardi-Vilardaga J. and Teixidor-Johe A. (1985). Embryonal hypermobility and articular development. Acta Anat. 123, 90-92. 10.1159/000146045 [DOI] [PubMed] [Google Scholar]
- Schittny J. C., Miserocchi G. and Sparrow M. P. (2000). Spontaneous peristaltic airway contractions propel lung liquid through the bronchial tree of intact and fetal lung explants. Am. J. Respir. Cell Mol. Biol. 23, 11-18. 10.1165/ajrcmb.23.1.3926 [DOI] [PubMed] [Google Scholar]
- Schwartz A. G., Pasteris J. D., Genin G. M., Daulton T. L. and Thomopoulos S. (2012). Mineral distributions at the developing tendon enthesis. PLoS ONE 7, e48630 10.1371/journal.pone.0048630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. G., Long F. and Thomopoulos S. (2015). Enthesis fibrocartilage cells originate from a population of Hedgehog-responsive cells modulated by the loading environment. Development 142, 196-206. 10.1242/dev.112714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer R., Chyung J. H., Murtaugh L. C., Brent a E., Rosen V., Olson E. N., Lassar A. and Tabin C. J. (2001). Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128, 3855-3866. [DOI] [PubMed] [Google Scholar]
- Schweitzer R., Zelzer E. and Volk T. (2010). Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development 137, 3347-3347. 10.1242/dev.057885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H.-S. and Serra R. (2007). Deletion of Tgfbr2 in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints. Dev. Biol. 310, 304-316. 10.1016/j.ydbio.2007.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharir A., Stern T., Rot C., Shahar R. and Zelzer E. (2011). Muscle force regulates bone shaping for optimal load-bearing capacity during embryogenesis. Development 138, 3247-3259. 10.1242/dev.063768 [DOI] [PubMed] [Google Scholar]
- Shefelbine S. J. and Carter D. R. (2004). Mechanobiological predictions of growth front morphology in developmental hip dysplasia. J. Orthop. Res. 22, 346-352. 10.1016/j.orthres.2003.08.004 [DOI] [PubMed] [Google Scholar]
- Shwartz Y., Farkas Z., Stern T., Aszodi A., Zelzer E., Aszódi A. and Zelzer E. (2012). Muscle contraction controls skeletal morphogenesis through regulation of chondrocyte convergent extension. Dev. Biol. 370, 154-163. 10.1016/j.ydbio.2012.07.026 [DOI] [PubMed] [Google Scholar]
- Shwartz Y., Blitz E. and Zelzer E. (2013). One load to rule them all: mechanical control of the musculoskeletal system in development and aging. Differentiation 86, 104-111. 10.1016/j.diff.2013.07.003 [DOI] [PubMed] [Google Scholar]
- Shwartz Y., Viukov S., Krief S. and Zelzer E. (2016). Joint development involves a continuous influx of Gdf5-positive cells. Cell Rep. 15, 2577-2587. 10.1016/j.celrep.2016.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnoli A., O'Rear L., Chandler R. L., Granero-Molto F., Mortlock D. P., Gorska A. E., Weis J. A., Longobardi L., Chytil A., Shimer K. et al. (2007). TGF-β signaling is essential for joint morphogenesis. J. Cell Biol. 177, 1105-1117. 10.1083/jcb.200611031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Später D., Hill T. P., Gruber M. and Hartmann C. (2006). Role of canonical Wnt-signalling in joint formation. Eur. Cell Mater. 12, 71-80. 10.22203/eCM.v012a09 [DOI] [PubMed] [Google Scholar]
- Stern T., Aviram R., Rot C., Galili T., Sharir A., Kalish Achrai N., Keller Y., Shahar R. and Zelzer E. (2015). Isometric scaling in developing long bones is achieved by an optimal epiphyseal growth balance. PLoS Biol. 13, e1002212 10.1371/journal.pbio.1002212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale F. E. (1992). Myogenic cell lineages. Dev. Biol. 154, 284-298. 10.1016/0012-1606(92)90068-R [DOI] [PubMed] [Google Scholar]
- Storm E. E. and Kingsley D. M. (1996). Joint patterning defects caused by single and double mutations in members of the bone morphogenetic protein (BMP) family. Development 122, 3969-3079. [DOI] [PubMed] [Google Scholar]
- Storm E. E., Huynh T. V., Copeland N. G., Jenkins N. A., Kingsley D. M. and Lee S.-J. (1994). Limb alterations in brachypodism mice due to mutations in a new member of the TGFβ-superfamily. Nature 368, 639-643. 10.1038/368639a0 [DOI] [PubMed] [Google Scholar]
- Subramanian A. and Schilling T. F. (2014). Thrombospondin-4 controls matrix assembly during development and repair of myotendinous junctions. Elife 2014, 3, e02372 10.7554/eLife.02372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A. and Schilling T. F. (2015). Tendon development and musculoskeletal assembly: emerging roles for the extracellular matrix. Development 142, 4191-4204. 10.1242/dev.114777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y., Takimoto A., Akiyama H., Kist R., Scherer G., Nakamura T., Hiraki Y. and Shukunami C. (2013). Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development 140, 2280-2288. 10.1242/dev.096354 [DOI] [PubMed] [Google Scholar]
- Summers A. P. and Koob T. J. (2002). The evolution of tendon–morphology and material properties. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 133, 1159-1170. 10.1016/S1095-6433(02)00241-6 [DOI] [PubMed] [Google Scholar]
- Suzue T. (1996). Movements of mouse fetuses in early stages of neural development studied in vitro. Neurosci. Lett. 218, 131-134. 10.1016/S0304-3940(96)13141-4 [DOI] [PubMed] [Google Scholar]
- Swinehart I. T., Schlientz A. J., Quintanilla C. A., Mortlock D. P. and Wellik D. M. (2013). Hox11 genes are required for regional patterning and integration of muscle, tendon and bone. Development 140, 4574-4582. 10.1242/dev.096693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto A., Kawatsu M., Yoshimoto Y., Kawamoto T. and Seiryu M. (2015). Scleraxis and osterix antagonistically regulate tensile force-responsive remodeling of the periodontal ligament and alveolar bone. Development 142, 787-796. 10.1242/dev.116228 [DOI] [PubMed] [Google Scholar]
- Terai K., Takano-Yamamoto T., Ohba Y., Hiura K., Sugimoto M., Sato M., Kawahata H., Inaguma N., Kitamura Y. and Nomura S. (1999). Role of osteopontin in bone remodeling caused by mechanical stress. J. Bone Miner. Res. 14, 839-849. 10.1359/jbmr.1999.14.6.839 [DOI] [PubMed] [Google Scholar]
- Thomopoulos S. (2011). The role of mechanobiology in the attachment of tendon to bone. IBMS Bonekey 8, 271-285. 10.1138/20110515 [DOI] [Google Scholar]
- Thomopoulos S., Genin G. M. and Galatz L. M. (2010). The development and morphogenesis of the tendon-to-bone insertion - what development can teach us about healing. J. Musculoskelet. Neuronal Interact. 10, 35-45. [PMC free article] [PubMed] [Google Scholar]
- Thompson, D. W. (1917). On Growth and Form, 1st edn. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Tickle C. (2015). How the embryo makes a limb: determination, polarity and identity. J. Anat. 227, 418-430. 10.1111/joa.12361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tintignac L. A., Brenner H.-R. and Rüegg M. A. (2015). Mechanisms regulating neuromuscular junction development and function and causes of muscle wasting. Physiol. Rev. 95, 809-852. 10.1152/physrev.00033.2014 [DOI] [PubMed] [Google Scholar]
- Tomlinson R. E., Li Z., Zhang Q., Goh B. C., Li Z., Thorek D. L. J., Rajbhandari L., Brushart T. M., Minichiello L., Zhou F. et al. (2016). NGF-TrkA signaling by sensory nerves coordinates the vascularization and ossification of developing endochondral bone. Cell Rep. 16, 2723-2735. 10.1016/j.celrep.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia M., Vega-macaya F. and Olguín P. (2017). Mechanical control of myotendinous junction formation and tendon differentiation during development. Front. Cell Dev. Biol. 5, 1-8. 10.3389/fcell.2017.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilyev A., Liu Y., Mudumana S., Mangos S., Lam P.-Y., Majumdar A., Zhao J., Poon K.-L., Kondrychyn I., Korzh V. et al. (2009). Collective cell migration drives morphogenesis of the kidney nephron. PLoS Biol. 7, e1000009 10.1371/journal.pbio.1000009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit G., Hansen U., Keene D. R., Bruckner P., Chiquet-Ehrismann R., Chiquet M. and Koch M. (2006). Collagen XII interacts with avian tenascin-X through its NC3 domain. J. Biol. Chem. 281, 27461-27470. 10.1074/jbc.M603147200 [DOI] [PubMed] [Google Scholar]
- Vortkamp A., Lee K., Lanske B., Segre G. V., Kronenberg H. M. and Tabin C. J. (1996). Regulation of rate of cartilage differentiation by Indian Hedgehog and PTH-related protein. Science 273, 613-622. 10.1126/science.273.5275.613 [DOI] [PubMed] [Google Scholar]
- Waggett A. D., Benjamin M. and Ralphs J. R. (2006). Connexin 32 and 43 gap junctions differentially modulate tenocyte response to cyclic mechanical load. Eur. J. Cell Biol. 85, 1145-1154. 10.1016/j.ejcb.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Wang X. and Mao J. J. (2002). Chondrocyte proliferation of the cranial base cartilage upon in vivo mechanical stresses. J. Dent. Res. 81, 701-705. 10.1177/154405910208101009 [DOI] [PubMed] [Google Scholar]
- Wang M., Vanhouten J. N., Nasiri A. R., Johnson R. L. and Broadus A. E. (2013). PTHrP regulates the modeling of cortical bone surfaces at fibrous insertion sites during growth. J. Bone Miner. Res. 28, 598-607. 10.1002/jbmr.1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washabaugh C. H., Ontell M. P., Shan Z., Hoffman E. P. and Ontell M. (1998). Role of the nerve in determining fetal skeletal muscle phenotype. Dev. Dyn. 211, 177-190. [DOI] [PubMed] [Google Scholar]
- Washabaugh C. H., Ontell M. P., Shand S. H., Bradbury N., Kant J. A. and Ontell M. (2007). Neuronal control of myogenic regulatory factor accumulation in fetal muscle. Dev. Dyn. 236, 732-745. 10.1002/dvdy.21078 [DOI] [PubMed] [Google Scholar]
- Weitkunat M., Kaya-Çopur A., Grill S. W. and Schnorrer F. (2014). Tension and force-resistant attachment are essential for myofibrillogenesis in Drosophila flight muscle. Curr. Biol. 24, 705-716. 10.1016/j.cub.2014.02.032 [DOI] [PubMed] [Google Scholar]
- Weitkunat M., Brasse M., Bausch A. R. and Schnorrer F. (2017). Mechanical tension and spontaneous muscle twitching precede the formation of cross-striated muscle in vivo. Development 144, 1261-1272. 10.1242/dev.140723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M., Siegrist M. and Goodwin K. (2003). Cyclic tensile strain and cyclic hydrostatic pressure differentially regulate expression of hypertrophic markers in primary chondrocytes. Bone 33, 685-693. 10.1016/S8756-3282(03)00242-4 [DOI] [PubMed] [Google Scholar]
- Wu Q.-Q. and Chen Q. (2000). Mechanoregulation of chondrocyte proliferation, maturation, and hypertrophy: ion-channel dependent transduction of matrix deformation signals. Exp. Cell Res. 256, 383-391. 10.1006/excr.2000.4847 [DOI] [PubMed] [Google Scholar]
- Wu Q.-Q., Zhang Y. and Chen Q. (2001). Indian hedgehog is an essential component of mechanotransduction complex to stimulate chondrocyte proliferation. J. Biol. Chem. 276, 35290-35296. 10.1074/jbc.M101055200 [DOI] [PubMed] [Google Scholar]
- Yang X., Arber S., William C., Li L., Tanabe Y., Jessell T. M., Birchmeier C. and Burden S. J. (2001). Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron 30, 399-410. 10.1016/S0896-6273(01)00287-2 [DOI] [PubMed] [Google Scholar]
- Zelená J. and Soukup T. (1983). The in-series and in-parallel components in rat hindlimb tendon organs. Neuroscience 9, 899-910. 10.1016/0306-4522(83)90278-6 [DOI] [PubMed] [Google Scholar]
- Zelzer E., Blitz E., Killian M. L. and Thomopoulos S. (2014). Tendon-to-bone attachment: From development to maturity. Birth Defects Res. Part C Embryo Today Rev. 102, 101-112. 10.1002/bdrc.21056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Liang C., Bates R., Yin Y., Xiong W.-C. and Mei L. (2012). Wnt proteins regulate acetylcholine receptor clustering in muscle cells. Mol. Brain 5, 7 10.1186/1756-6606-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K., Shen C., Lu Y., Huang Z., Li L., Rand C. D., Pan J., Sun X.-D., Tan Z., Wang H. et al. (2017). Muscle Yap is a regulator of neuromuscular junction formation and regeneration. J. Neurosci. 37, 3465-3477. 10.1523/JNEUROSCI.2934-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W., Li Y., Li L., Zhang W., Wang S. and Zheng X. (2013). YAP-mediated regulation of the chondrogenic phenotype in response to matrix elasticity. J. Mol. Histol. 44, 587-595. 10.1007/s10735-013-9502-y [DOI] [PubMed] [Google Scholar]