Abstract
Hoxa5 is essential for development of several organs and tissues. In the respiratory system, loss of Hoxa5 function causes neonatal death due to respiratory distress. Expression of HOXA5 protein in mesenchyme of the respiratory tract and in phrenic motor neurons of the central nervous system led us to address the individual contribution of these Hoxa5 expression domains using a conditional gene targeting approach. Hoxa5 does not play a cell-autonomous role in lung epithelium, consistent with lack of HOXA5 expression in this cell layer. In contrast, ablation of Hoxa5 in mesenchyme perturbed trachea development, lung epithelial cell differentiation and lung growth. Further, deletion of Hoxa5 in motor neurons resulted in abnormal diaphragm innervation and musculature, and lung hypoplasia. It also reproduced the neonatal lethality observed in null mutants, indicating that the defective diaphragm is the main cause of impaired survival at birth. Thus, Hoxa5 possesses tissue-specific functions that differentially contribute to the morphogenesis of the respiratory tract.
KEY WORDS: Hoxa5, Respiratory system development, Lung, Trachea, Diaphragm, Mouse
Summary: Characterization of Hoxa5 conditional mutant mice establishes that Hoxa5 regulates respiratory system development through distinct mechanisms in epithelium, mesenchyme and phrenic motor neurons.
INTRODUCTION
In mammals, successful transition to air breathing at birth requires harmonious development of the respiratory system, a highly orchestrated process that involves the synchronized formation of trachea, lung and diaphragm. In the mouse, respiratory development initiates around embryonic day (E) 9 with the outpocketing and elongation of the foregut endoderm into the surrounding mesenchyme to form the laryngotracheal groove and primary lung buds (Morrisey and Hogan, 2010). Next, a coordinated program of dichotomous branching gives rise to the airway tree. Transition from branching morphogenesis to differentiation of the respiratory epithelium into specialized cell types, regionally distributed along the proximo-distal axis, leads to a patterned respiratory tract (Chang et al., 2013). Functional air–blood barriers form via differentiation of the distal epithelium in close association with the expansion of the vasculature. Lung development ends after birth with formation of alveoli, which increase the gas-exchange surface area to meet the respiratory requirements of the growing organism.
Physical forces and mechanical interactions between the thorax and respiratory tract are involved in lung growth and function. In fluid-filled fetal lungs, distention is maintained by breathing-like movements and by upper airway resistance during apnea (Kotecha, 2000). Genetic defects that perturb the thoracic skeleton, or space-occupying lesions that compress the lungs, like congenital diaphragmatic hernia, can cause pulmonary hypoplasia; this in turn compromises respiratory function and can be lethal (Jay et al., 2007). Lung development also requires innervation of the diaphragm by the phrenic nerves. Diaphragm denervation abolishes fetal breathing movements, which impairs lung growth (Wigglesworth and Desai, 1979; Liggins et al., 1981).
Diaphragm muscle formation involves contributions from several embryonic structures: somites, pleuroperitoneal folds (PPFs), septum transversum and neural tube (Merrell and Kardon, 2013; Merrell et al., 2015). Around E10.5, muscle progenitors delaminate from the hypaxial dermomyotome of the cervical (C) somites C3 to C5 to reach the PPFs, proliferate and spread ventrally and dorsally on the septum transversum to colonize the entire diaphragm, except the tendinous central region. Muscle progenitors then undergo two waves of myogenesis to differentiate into myofibers (Bentzinger et al., 2012). Meanwhile, the phrenic motor neurons exit the neural tube, also from the C3-C5 region, reach the PPFs and split into three primary trunks, the sternocostal, dorsocostal and crural branches, to innervate the entire diaphragm (Merrell and Kardon, 2013). Muscle progenitors and nerves reach the PPFs at the cervical level, and together descend toward the lower thorax as the lung and heart grow within the thoracic cavity (Allan and Greer, 1997). The PPFs, which constitute a transient embryonic structure, ultimately contribute to the diaphragm's central tendon and connective tissue fibroblasts (Merrell et al., 2015).
Respiratory tract morphogenesis relies on the functional integration of several transcriptional regulators and signaling pathways. Together, they direct reciprocal interactions between epithelium and mesenchyme, and control cell proliferation, differentiation and branching (Morrisey and Hogan, 2010; Hogan et al., 2014). Inappropriate expression of regulatory genes can cause malformations and diseases.
In vertebrates, Hox genes occupy a crucial position in the developmental hierarchy. They encode transcription factors that control the formation of body segment-specific structures via downstream effectors that, in turn, direct region-specific morphogenetic events in numerous tissue types along the embryonic axes. In mammals, 39 Hox genes are organized in four clusters and classified into 13 paralog groups. The spatiotemporal profile of Hox expression reflects the organization of clusters, the 3′ most genes being expressed earlier and in more anterior domains than the 5′ located ones (McGinnis and Krumlauf, 1992). Several Hox genes, predominantly from the 3′ half of clusters, are expressed along the respiratory tract, each with distinct proximal-distal distribution (Herriges et al., 2012). Previous work has shown the requirement for the three Hox5 paralog genes in lung development. They are expressed in a partially overlapping pattern: Hoxa5 and Hoxb5 are strongly expressed in lung mesenchyme, whereas Hoxc5 expression is barely detected (Boucherat et al., 2013; Hrycaj et al., 2015). Hoxa5 and Hoxc5, but not Hoxb5, are expressed in the phrenic motor neurons, which innervate the diaphragm (Philippidou et al., 2012). Finally, only Hoxa5 is expressed in tracheal mesenchyme. In agreement with its numerous expression domains in the developing respiratory system, most Hoxa5−/− pups die at birth from respiratory failure, and show tracheal occlusion, lung hypoplasia and cell misspecification, and diaphragm innervation defects (Jeannotte et al., 1993; Aubin et al., 1997; Boucherat et al., 2013). In contrast, Hoxb5 and Hoxc5 single mutants are viable. The lung phenotypic traits of Hoxb5 mutants are less severe than for Hoxa5 mutants, whereas Hoxc5 mutants lack respiratory defects (Boucherat et al., 2013; P.P. and L.J., unpublished). These data unveil the functional predominance of Hoxa5 in formation of the respiratory system. They also show that Hoxa5 can compensate for the lack of Hox5 paralog function, as further supported by the increased Hoxa5 expression detected in Hoxb5−/− lungs (Boucherat et al., 2013). Compound mutants for the three Hox5 genes display an exacerbated lung phenotype, likely reflecting functional redundancy due to expression overlap (Hrycaj et al., 2015).
The functions of Hoxa5 in trachea, lung and diaphragm may separately or in combination affect lung formation and neonate survival. To dissect the tissue-specific role(s) of Hoxa5 in respiratory system development, we used a Hoxa5 conditional allele with Cre recombinase deleter strains to ablate Hoxa5 in epithelium, mesenchyme, or motor neurons. Epithelial deletion revealed no role for Hoxa5 in this tissue, but deletion in either of the latter tissues resulted in hypoplastic lungs. Mesenchymal deletion of Hoxa5 also reproduced the abnormal patterning of tracheal cartilage and lung cell misspecification seen in Hoxa5 null mutants. However, only deletion of Hoxa5 in motor neurons caused death at birth owing to the abnormal innervation and formation of the diaphragm. Thus, the functional prevalence of Hoxa5 in the formation of the respiratory system results from distinct functions of Hoxa5 in its different expression sites.
RESULTS
HOXA5 expression in the developing respiratory system
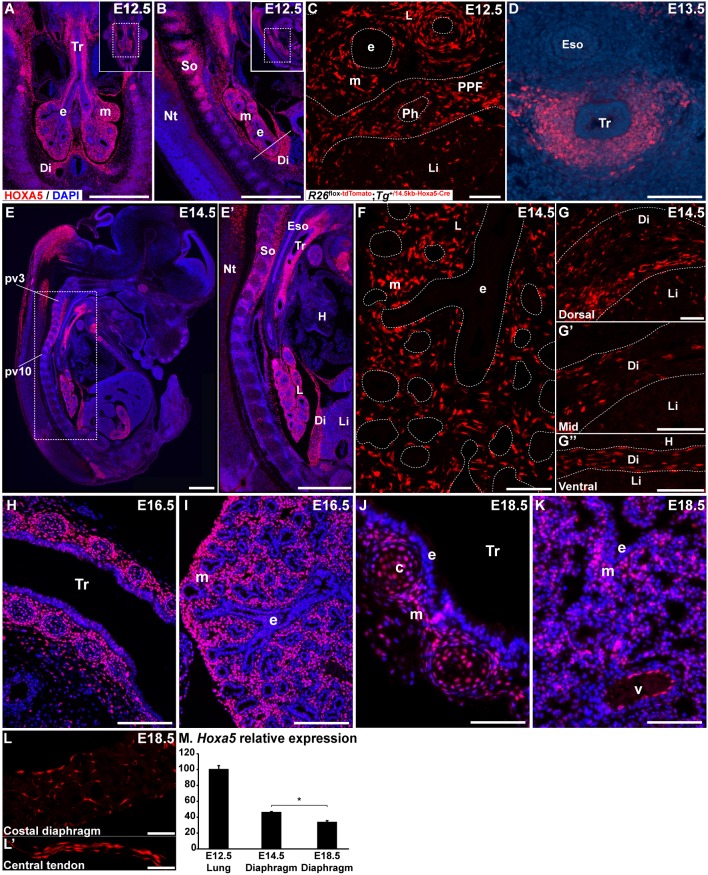
We previously showed that Hoxa5 mRNA is expressed in the mesenchyme along the entire embryonic respiratory tract (Boucherat et al., 2013). Immunofluorescence assays confirmed HOXA5 protein expression in mesenchymal nuclei of upper airways and distal lung at E12.5 during branching morphogenesis, and also in the developing diaphragm (Fig. 1A,B). Further, descendants of Hoxa5-expressing cells showed restricted fates within the diaphragm. We bred a Hoxa5-Cre transgenic line with the R26mT reporter mouse. R26mT-positive cells, corresponding to descendants of Hoxa5-expressing cells, were detected in the PPF region of the diaphragm (Bérubé-Simard and Jeannotte, 2014) (Fig. 1C). In the trachea and primary bronchi, HOXA5 protein expression was observed in the ventrolateral mesenchyme (Fig. 1D). HOXA5 distribution in lung and trachea remained similar at later stages (Fig. 1E-F,H-K). As previously shown, HOXA5 immunoreactivity was detected in the prevertebra (pv)3-pv10 region encompassing the domain where diaphragm muscle progenitors originate and phrenic motor column axons exit and project to contact the diaphragm (Fig. 1E,E′) (Coulombe et al., 2010). Hoxa5-expressing cells were also present throughout the diaphragm at E14.5 (Fig. 1G-G″). At the end of gestation, Hoxa5 expression was reduced in the diaphragm, and descendants of Hoxa5-expressing cells were observed in the costal region and central tendon (Fig. 1L-M). No co-labeling between Hoxa5-expressing or descendant cells and cells positive for the myogenic transcriptional regulator PAX7 was detected in the developing diaphragm, indicating that Hoxa5 was not expressed in the diaphragm muscle lineage (Fig. S1). In trachea, HOXA5 expression was detected in cartilage condensations and the surrounding mesenchyme at E16.5 and E18.5. In lung, HOXA5 mesenchymal expression became sparser (Fig. 1H-K). HOXA5 protein was also detected in pulmonary endothelial cells (Fig. 1K). HOXA5 expression was not detected in and Hoxa5 descendants were absent from respiratory epithelium at all stages.
Fig. 1.
HOXA5 protein expression in the respiratory system. (A,B,D-E′,H-K) HOXA5 protein was detected by immunofluorescence in mesenchymal nuclei from the trachea, lung and developing diaphragm at E12.5 (A,B), E13.5 (D), E14.5 (E,E′), E16.5 (H,I) and E18.5 (J,K). At E16.5 (H) and E18.5 (J), HOXA5 protein was present in tracheal cartilage cells and surrounding mesenchyme. HOXA5 was also observed in endothelial cells lining lung blood vessels (K). (C,F-G″,L,L′) Cell lineage analysis of R26mT; Hoxa5-Cre embryos showed R26mT-positive cells in lung mesenchyme at E12.5 (C) and E14.5 (F), in the PPF region of developing diaphragm at E12.5 (C) and in diaphragm at E14.5 (G-G″) and E18.5 (L,L′). Frontal (A,H-K), sagittal (B,E-G″,L,L′) and transverse (C,D) sections are shown. The white line in B represents the sectional plan of the PPF region in C. (M) qRT-PCR analysis of Hoxa5 expression in E12.5 lung and in diaphragm at E14.5 and E18.5. Mean±s.e.m. are shown. *P<0.05 (Student's t-test). c, cartilage; Di, diaphragm; e, epithelium; Eso, esophagus; H, heart; L, lung; Li, liver; m, mesenchyme; Nt, neural tube; Ph, phrenic nerve; pv, prevertebra; So, somites; Tr, trachea; v, vessel. Scale bars: 500 µm (A,B,E,E′); 100 µm (H); 50 µm (C,D,F-G″,I-K); 25 µm (L,L′).
No cell-autonomous role for Hoxa5 in respiratory epithelium
Although HOXA5 expression appeared to be absent from lung epithelium, it remains possible that expression is too low or restricted for a small cell population to be detected. To exclude a cell-autonomous function in lung epithelium, we ablated Hoxa5 in epithelium with the Shhcre line (Harfe et al., 2004; Boucherat et al., 2014). Immunohistochemistry (IHC) confirmed that HOXA5 expression was not altered in trachea, lung and diaphragm of Hoxa5flox/flox; Shh+/Cre specimens (Fig. S2D-I). Further, no phenotype was apparent in these specimens. Offspring were obtained at expected Mendelian ratios at E18.5 and this ratio was maintained to adulthood (Fig. S3A). No lung hypoplasia was observed (Fig. S3B). Epithelial cell differentiation was normal, as assessed by the abundance and distribution of secretory club cells and type I pneumocytes, two cell types that are reduced in Hoxa5−/− mutants (Fig. S3C-F). Other Hoxa5 null phenotypes, including goblet cell metaplasia and enlarged lung airspaces caused by perturbed myofibroblast localization, were also absent following Hoxa5 epithelial deletion (Fig. S3G-J) (Mandeville et al., 2006). Altogether, these data confirmed that Hoxa5 has no cell-autonomous role in respiratory epithelium.
Hoxa5 expression in phrenic motor neurons is crucial for survival at birth
To assess the requirement for Hoxa5 in respiratory mesenchyme and phrenic motor neurons, we used the Dermo1+/cre and Olig2+/cre deleter mouse lines, respectively (Yu et al., 2003; Dessaud et al., 2007; Dermo1 is also known as Twist2). Dermo1Cre activity is specific to lung and tracheal mesenchyme (Boucherat et al., 2014). However, the Dermo1Cre allele was not effective in the entire developing diaphragm, demonstrated by the lack of reporter staining in the PPFs (Fig. S2A). Cre activity of the Olig2+/cre line was restricted to the ventral spinal cord with no expression in skeletal muscle or lung (Fig. S2B,C) (Park et al., 2010a). Olig2Cre efficiently removes Hoxa5 from motor neurons (Philippidou et al., 2012). Here, we confirmed by IHC and quantitative RT-PCR (qRT-PCR) that it did not alter Hoxa5 expression in other respiratory tissues (Fig. S2J-L,X,Y). In Hoxa5flox/flox; Dermo1+/Cre embryos, HOXA5 expression was reduced but not abolished in trachea, lung and diaphragm mesenchyme, indicating that the Dermo1Cre was inefficient or not expressed in all mesenchymal cell types (Fig. S2M-O,X,Y). To reduce mesenchymal Hoxa5 expression further, we generated Hoxa5flox/−; Dermo1+/Cre animals. Near-complete loss of HOXA5 protein expression was observed in the trachea and lung from these embryos, but it was still detected in the diaphragm (Fig. S2P-Y).
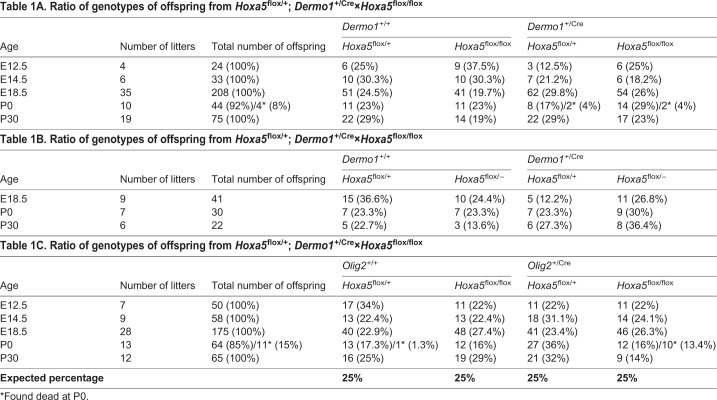
Hoxa5flox/flox; Dermo1+/Cre and Hoxa5flox/−; Dermo1+/Cre individuals were obtained at expected Mendelian ratios at embryonic and adult ages, suggesting either that the mortality of Hoxa5−/− pups did not result from the loss of Hoxa5 mesenchymal expression or that residual Hoxa5 expression was sufficient to allow survival (Table 1A,B). Conversely, 45% of the Hoxa5flox/flox;Olig2+/Cre newborns became cyanotic, developed respiratory distress and died within 24 h of birth, indicating that Hoxa5 deletion in motor neurons was sufficient to reproduce the mortality rate observed for Hoxa5−/− pups (Table 1C).
Table 1A.
Ratio of genotypes of offspring from Hoxa5flox/+; Dermo1+/Cre×Hoxa5flox/flox
Tracheal cartilage patterning requires Hoxa5 in mesenchyme
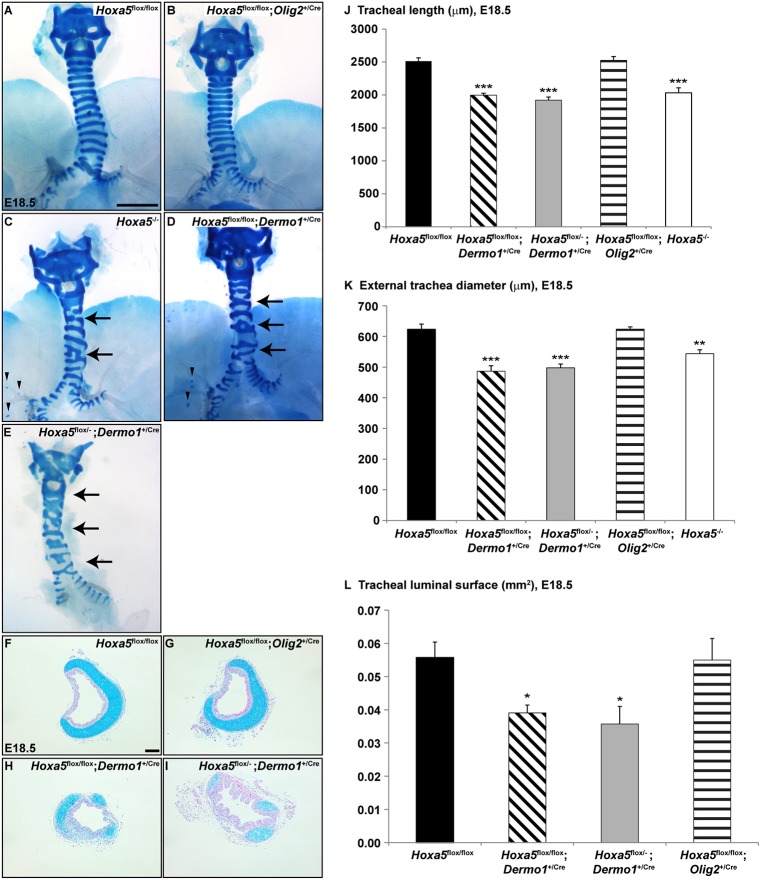
Although Hoxa5 mesenchymal inactivation was incomplete, it recapitulated several aspects of the Hoxa5 null phenotype, indicating that a threshold of Hoxa5 expression is required in the mesenchyme. For example, defects in tracheal cartilage rings are fully penetrant in Hoxa5−/− mice (Aubin et al., 1997). Tracheas from controls and Hoxa5flox/flox; Olig2+/Cre were normal (Fig. 2A,B,F,G). Conversely, all Hoxa5flox/flox; Dermo1+/Cre and Hoxa5flox/−; Dermo1+/Cre trachea specimens displayed irregular and incompletely formed cartilaginous rings with abnormal lateral fusion and merging of the cricoid cartilage with the first cartilage rings, anomalies that phenocopy those encountered in Hoxa5−/− mice (Fig. 2C-E,H,I). Mesenchymal deletion of Hoxa5 shared additional phenotypes with Hoxa5−/− embryos including ectopic cartilage dots in lungs (Fig. 2C,D), and reduced tracheal length, external diameter and luminal surface (Fig. 2J-L) (Boucherat et al., 2013). Thus, Hoxa5 activity in mesenchyme, but not in motor neurons, is essential for tracheal cartilage patterning.
Fig. 2.
Mesenchymal Hoxa5 deletion causes abnormal tracheal cartilage patterning. (A-E) Alcian Blue staining of whole-mount E18.5 tracheas showed cartilage ring malformations (arrows) and ectopic cartilage points (arrowheads) in Hoxa5−/− and Hoxa5; Dermo1+/Cre mutants. (F-I) Cartilage defects and reduced luminal surface were demonstrated by Alcian Blue staining of transverse tracheal sections. (J-L) Morphometric measurements of tracheal length (J), external tracheal diameter (K) and luminal surface (L) were evaluated. Mean±s.e.m. are shown. *P<0.05, **P<0.01, ***P<0.001 (Student's t-test). Scale bars: 1 mm (A-E); 100 µm (F-I).
Hoxa5−/− embryos show disorganization of the tracheal epithelial layer at E18.5; however, no quantitative or qualitative variation in club, ciliated, basal and goblet cells was observed (Fig. S4) (Aubin et al., 1997). Smooth muscle cells of the trachealis muscle, derived from the dorsal mesenchyme, appeared normal as detected by αSMA (ACTA2) staining, consistent with lack of Hoxa5 expression in these cells (Fig. 1D, Fig. S4). Thus, Hoxa5 mesenchymal expression does not contribute to the dorso-ventral patterning of the trachea or to the epithelial-mesenchymal crosstalk that governs tracheal cell specification.
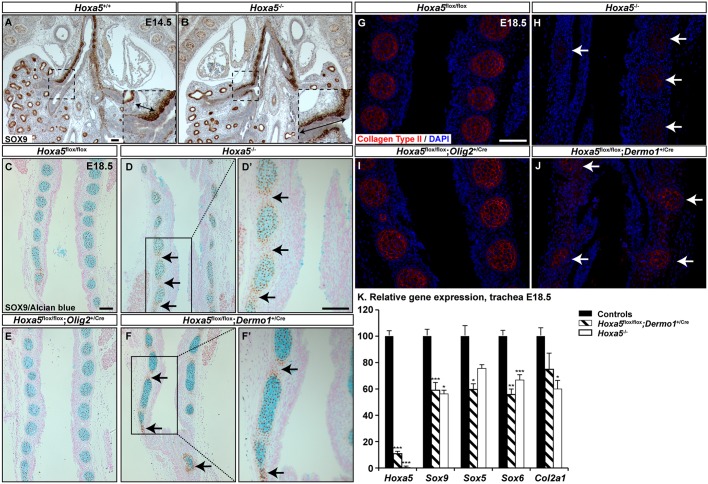
To characterize the mechanisms underlying the Hoxa5 tracheal phenotype, we examined expression of SOX9, a key transcriptional regulator of chondrogenesis (Turcatel et al., 2013). In the trachea, SOX9 is first uniformly expressed in the ventrolateral mesenchyme, and then expression becomes segmented in a pattern prefiguring the cartilage rings (Elluru and Whitsett, 2004). In lung, SOX9 expression is restricted to committed distal endodermal progenitor cells (Tian et al., 2011). In E14.5 Hoxa5−/− embryos, mesenchymal SOX9 protein expression expanded caudally along the secondary bronchi (Fig. 3A,B). This ectopic SOX9 expression might explain the abnormal cartilage dots in mutant lungs. At E18.5, SOX9 tracheal expression was limited to Alcian Blue-positive cartilage rings in controls and Hoxa5flox/flox; Olig2+/Cre specimens. In Hoxa5−/− and Hoxa5flox/flox; Dermo1+/Cre embryos, ectopic SOX9 expression was observed outside of the cartilaginous nodules (Fig. 3C-F′). Expression of cartilage-specific type II collagen, a direct transcriptional target of SOX9, was reduced in Hoxa5−/− and Hoxa5flox/flox; Dermo1+/Cre tracheas (Fig. 3G-J) (Lefebvre et al., 1997). qRT-PCR analysis confirmed the decrease in Col2a1 mRNA levels, which was more substantial in Hoxa5−/− specimens probably owing to the residual Hoxa5 expression in trachea of Hoxa5flox/flox; Dermo1+/Cre mutants (Fig. 3K). qRT-PCR also revealed a significant diminution of Sox9 expression levels, despite the spatial expansion observed. Consistent with this, expression of Sox5 and Sox6, which are genetically downstream of Sox9 in cartilage specification and constitute with Sox9 the chondrogenic regulator trio, was also decreased (Fig. 3K) (Lefebvre et al., 1998).
Fig. 3.
Mesenchymal Hoxa5 ablation affects SOX9 spatial expression in trachea. (A,B) SOX9 immunostaining showed ectopic mesenchymal SOX9 expression along the secondary bronchi of E14.5 Hoxa5−/− embryos. Insets show higher magnifications of the boxed areas. Double arrows indicate the SOX9 expression domain along the secondary bronchi. (C-F′) SOX9 expression was detected outside of the cartilage nodules in E18.5 Hoxa5−/− and Hoxa5flox/flox; Dermo1+/Cre mutants (arrows). (G-J) Collagen type II staining was reduced in Hoxa5−/− and Hoxa5flox/flox; Dermo1+/Cre mutants (arrows). (K) qRT-PCR analysis of Hoxa5, Sox9, Sox5, Sox6 and Col2a1 expression levels in E18.5 control and Hoxa5−/− tracheas. Mean±s.e.m. are shown. *P<0.05, **P<0.01, ***P<0.001 (Student's t-test). Scale bars: 100 µm.
To characterize further the molecular mechanisms leading to Sox9 dysregulation and cartilage defects, we next examined other known regulators of tracheal cartilage patterning: Fgf10, Shh, Bmp4, Tbx4 and Tbx5. Of these, all but Fgf10 influence Sox9 expression in tracheal mesenchyme (Arora et al., 2012; Park et al., 2010b; Sala et al., 2011). qRT-PCR showed significantly decreased expression of Bmp4 in the trachea from Hoxa5−/− and Hoxa5flox/flox; Dermo1+/Cre mutants, and similarly displayed reduction of Id1 and Id2 expression, which are targets of the BMP4 pathway (Fig. S5). Expression of the follistatin-like 1 (Fstl1) gene was also diminished in Hoxa5 mutants. Fstl1, which encodes a BMP antagonist, is a direct transcriptional target of HOXA5 in the trachea (Boucherat et al., 2013). No changes in Fgf10, Shh, Tbx4 and Tbx5 expression levels were detected (Fig. S5). These data suggest that Hoxa5 controls both spatial domain and expression levels of Sox9 in tracheal mesenchyme via mechanisms that involve BMP signaling. This regulation is cell-autonomous, because conditional deletion of Hoxa5 in mesenchyme recapitulates the null phenotype. Further, the incomplete deletion by Dermo1Cre indicates that a threshold level of Hoxa5 is required for its full activity in tracheal mesenchyme.
Lung hypoplasia results from loss of Hoxa5 in either mesenchyme or motor neurons
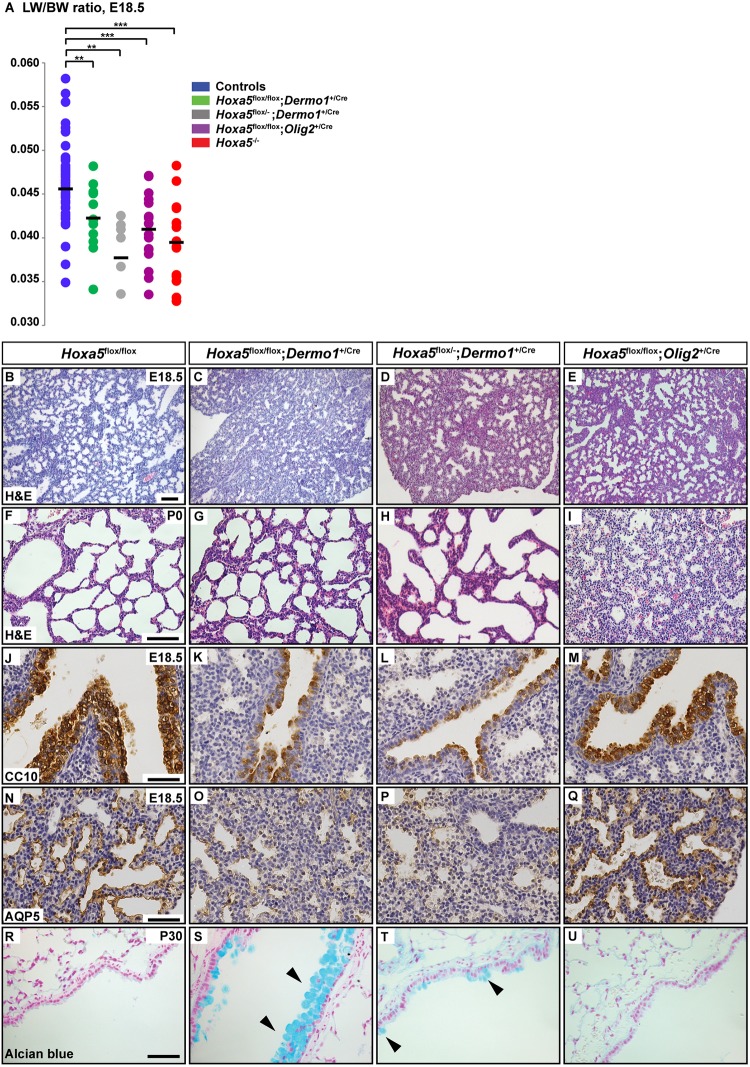
Lung hypoplasia, measured as a reduced lung weight/body weight (LW/BW) ratio, occurred in Hoxa5flox/flox; Dermo1+/Cre, Hoxa5flox/−; Dermo1+/Cre and Hoxa5flox/flox; Olig2+/Cre mutants and in Hoxa5−/− embryos (Fig. 4A). Decreased proliferation contributes to Hoxa5−/− lung hypoplasia (Boucherat et al., 2013). A similar downward trend was observed in the number of 5-bromo-2′-deoxyuridine (BrdU)-positive cells in Hoxa5flox/flox; Dermo1+/Cre, Hoxa5flox/−; Dermo1+/Cre and Hoxa5flox/flox; Olig2+/Cre lungs (Fig. S6). Hoxa5 is thus essential in both lung mesenchyme and phrenic motor neurons innervating the diaphragm to sustain correct proliferation of lung tissues.
Fig. 4.
Hoxa5 mesenchymal and motor neuron mutations differentially influence lung development. (A) The LW/BW ratio was significantly decreased in E18.5 Hoxa5flox/flox; Dermo1+/Cre, Hoxa5flox/−; Dermo1+/Cre, Hoxa5flox/flox; Olig2+/Cre and Hoxa5−/− embryos versus controls. **P<0.01, ***P<0.001 (Student's t-test). (B-E) Comparative histology of lung morphology showed that most affected E18.5 Hoxa5flox/flox; Dermo1+/Cre and Hoxa5flox/−; Dermo1+/Cre lungs exhibited narrower air spaces and thicker mesenchyme compared with Hoxa5flox/flox and Hoxa5flox/flox; Olig2+/Cre specimens. (F-I) At P0, Hoxa5flox/flox; Olig2+/Cre lungs were collapsed in contrast to Hoxa5flox/flox and Hoxa5; Dermo1+/Cre specimens. (J-Q) Correct differentiation of club cells and type I pneumocytes, as assessed by CC10 (also known as SCGB1A1) and AQP5 immunostaining, occurred in control and Hoxa5flox/flox; Olig2+/Cre specimens but reduced staining was observed in Hoxa5; Dermo1+/Cre specimens. (R-U) Goblet cells, detected by Alcian Blue staining, were scarce in control and Hoxa5flox/flox; Olig2+/Cre mice, whereas metaplasia was detected in Hoxa5flox/flox; Dermo1+/Cre and Hoxa5flox/−; Dermo1+/Cre upper airways (arrowheads). Scale bars: 100 µm (B-I); 50 µm (J-U).
Proper differentiation of lung epithelium requires Hoxa5 in mesenchyme
Hoxa5−/− embryos display abnormal and dense lung histology with airway epithelium differentiation defects (Boucherat et al., 2012, 2013). At E18.5, the most affected Hoxa5flox/flox; Dermo1+/Cre and Hoxa5flox/−; Dermo1+/Cre embryos similarly exhibited narrower air spaces and thicker mesenchyme (Fig. 4B-D). Control and Hoxa5flox/flox; Olig2+/Cre lung specimens presented a normal structure with dilated peripheral saccules and thin mesenchyme (Fig. 4B,E). At postnatal day (P) 0, thicker lung mesenchyme persisted in Hoxa5flox/flox; Dermo1+/Cre and Hoxa5flox/−; Dermo1+/Cre specimens but pulmonary structures were expanded (Fig. 4F-H). In contrast, lungs from Hoxa5flox/flox; Olig2+/Cre newborns found dead were collapsed compared with controls, indicating that neuronal Hoxa5 expression was essential for the expansion of the lung at birth (Fig. 4F,I).
Hoxa5 null mutation causes lung epithelial cell differentiation defects, including fewer club cells and type I pneumocytes (Boucherat et al., 2012, 2013). Both cell types were reduced in Hoxa5flox/flox; Dermo1+/Cre and Hoxa5flox/−; Dermo1+/Cre lungs, but not in control or Hoxa5flox/flox; Olig2+/Cre specimens (Fig. 4J-Q). Goblet cell metaplasia was also observed in Hoxa5flox/flox; Dermo1+/Cre and Hoxa5flox/−; Dermo1+/Cre adult mice whereas mucus-producing cells were scarce in controls and Hoxa5flox/flox; Olig2+/Cre mutants (Fig. 4R-U). Variable expressivity of the lung phenotype in specimens carrying the Dermo1Cre allele likely reflects the incomplete activity of the Dermo1Cre. Overall, the requirement for Hoxa5 in lung epithelial organization and differentiation appears to be mediated by Hoxa5 activity in mesenchyme, and does not require expression in motor neurons. This further illustrates that Hoxa5 possesses tissue-specific roles that differentially participate in respiratory tract morphogenesis.
Hoxa5 and Hoxb5 differentially affect lung regulatory signaling pathways
Deletion of all three Hox5 paralogs in the lung was reported to affect the canonical Wnt/Bmp4 signaling axis but not the Fgf and Shh pathways (Hrycaj et al., 2015). To assess the specific contribution of Hoxa5 in this context, we evaluated gene expression in Hoxa5−/− lungs by qRT-PCR. At E12.5, expression of Wnt2 and Wnt7a was reduced in Hoxa5−/− specimens (Fig. S7A). These are ligands of the canonical Wnt pathway expressed in lung mesenchyme and epithelium, respectively (Miller et al., 2012; Hrycaj et al., 2015). Wnt/β-catenin signaling activates the airway smooth muscle program, and acts upstream of Bmp4 and Fgf signaling to regulate differentiation of airway epithelium (Shu et al., 2005; Goss et al., 2011). In Hoxa5−/− lungs, expression of downstream targets of the canonical Wnt pathway was significantly decreased, including Lef1, a transcriptional effector of the pathway, Sm22a (Tagln), an early marker of the smooth muscle lineage, and Pdgfra, Fgf10, Bmp4 and Shh, key players in airway smooth muscle development and branching morphogenesis (Fig. S7A) (Morrisey and Hogan, 2010). At E18.5, expression of Wnt2, Wnt7a, Sm22a, Pdgfra and Fgf10 remained significantly diminished in Hoxa5−/− specimens (Fig. S7B). Analysis was also performed on E18.5 Hoxb5−/− and compound Hoxa5−/−; Hoxb5−/− mutant embryos. Similar reductions in Wnt2 and Fgf10 expression were observed in Hoxa5 and Hoxb5 single mutants and in compound mutants. Wnt7a expression was not affected in Hoxb5−/− specimens but was decreased in Hoxa5−/−; Hoxb5−/− mutants suggesting a predominant contribution of Hoxa5 to Wnt7a expression. In contrast, Shh and Lef1 expression was significantly diminished in Hoxb5−/− and Hoxa5−/−; Hoxb5−/− mutants. As in Hoxa5−/− lungs, Bmp4 expression was unaffected in Hoxb5−/− and Hoxa5−/−; Hoxb5−/− mutants. Finally, Pdgfra, Sm22a and Acta2 (encoding αSMA) expression was reduced in Hoxa5−/− and Hoxa5−/−; Hoxb5−/− but not in Hoxb5−/− mutant lungs. Thus, Hoxa5 and Hoxb5 genes contribute differentially to fine-tune the signaling networks controlling lung development. They act similarly on the Fgf10 pathway, their influence differs on Wnt/β-catenin and Shh signaling, and Hoxa5 specifically controls the lung airway smooth muscle program via regulation of Pdgfra.
Diaphragm innervation requires Hoxa5 expression in motor neurons
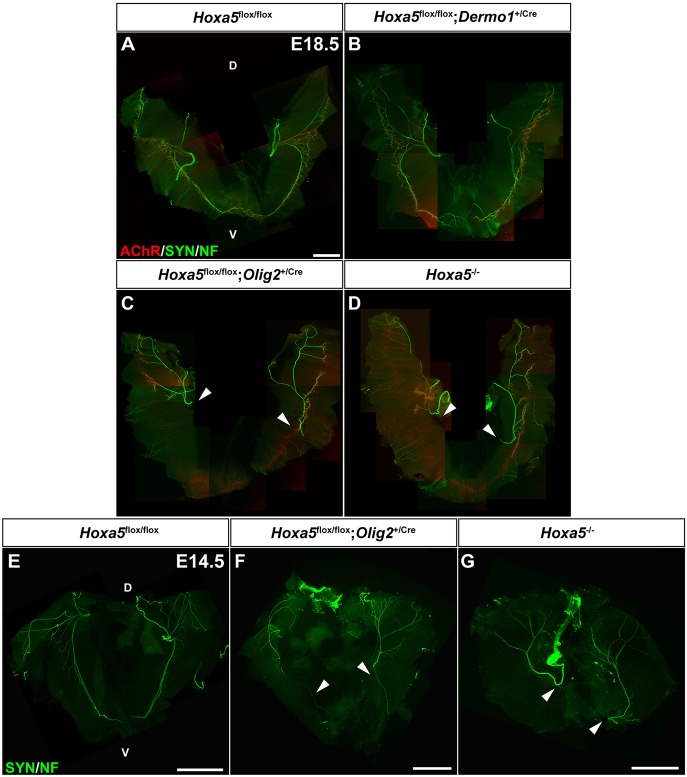
Almost half of the Hoxa5flox/flox; Olig2+/cre pups died at birth from respiratory distress with compact lungs and a significantly reduced LW/BW ratio, suggesting that lungs failed to grow and inflate normally as a result of impaired fetal breathing movements caused by abnormal diaphragm function. To confirm that this phenotype was associated with abnormal innervation of diaphragm, we characterized phrenic nerves of Hoxa5flox/flox; Olig2+/Cre embryos. The phrenic nerve arises bilaterally from motor neurons in the ventral horns of the cervical spinal cord and contacts the developing diaphragm near the mid-costal region before branching into three primary trunks, of which two extend to innervate the ventral and dorsal parts of diaphragm (Merrell and Kardon, 2013). Whole-mount staining of nerves and neuromuscular junctions revealed that, at E18.5, Hoxa5−/− and Hoxa5flox/flox; Olig2+/Cre embryos presented a severely compromised motor innervation pattern. Axons consistently failed to reach the ventral-most part of the costal muscle, and fewer branches were observed in the dorsal region. Diaphragm innervation was normal in controls and Hoxa5flox/flox; Dermo1+/Cre specimens (Fig. 5A-D). The phenotype was similar at E14.5 suggesting that altered axon patterning, rather than neuronal degeneration, was the cause of the defective innervation (Fig. 5E-G).
Fig. 5.
Abnormal diaphragm innervation in Hoxa5 motor neuron mutants. (A-G) Analysis of diaphragm innervation patterns at E18.5 (A-D) and E14.5 (E-G) by whole-mount IF staining of neurofilaments (NF), synaptophysin (SYN) and α-bungarotoxin (AChR). Arrowheads indicate failure of phrenic nerves to reach the most ventral part of diaphragm costal muscle at both ages in Hoxa5flox/flox; Olig2+/Cre and Hoxa5−/− specimens. Single images covering overlapping portions of the diaphragm muscle were manually stitched together in Photoshop to obtain the composite images of the entire muscle. D, dorsal; V, ventral. Scale bars: 1 mm.
Diaphragm muscle formation needs Hoxa5 expression in motor neurons
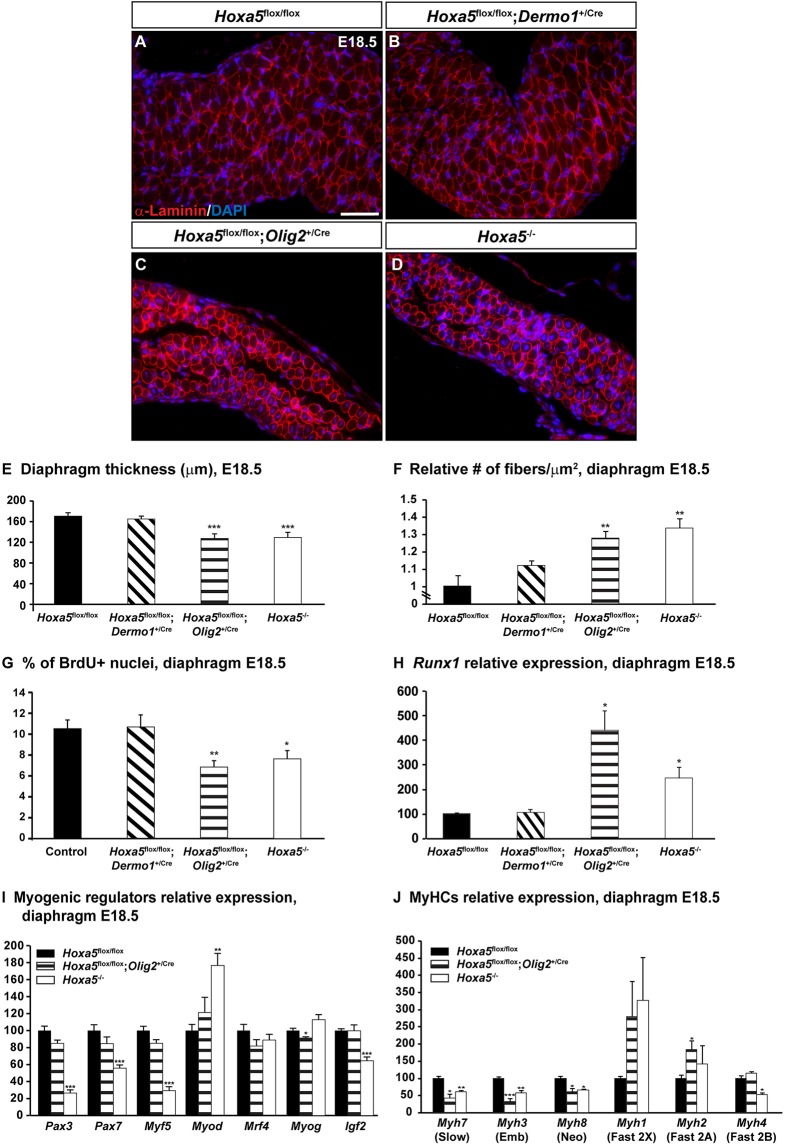
Axons typically regulate diaphragm muscle development from the time of initial contact, by facilitating myotube formation via electrically mediated effects and/or diffusible substances. We therefore analyzed the structure of diaphragm muscle fibers in E18.5 embryos by electron microscopy (Greer et al., 1999). Controls and Hoxa5flox/flox; Dermo1+/Cre diaphragms showed aligned sarcomeres. Conversely, Hoxa5−/− and Hoxa5flox/flox; Olig2+/Cre mutant specimens presented misaligned Z-discs, damaged sarcomeric proteins and much wider actin-only I-bands, indicative of a high proportion of non-contracted sarcomeres. These defects were observed in the entire muscularized diaphragm (Fig. 6). Further, at E18.5, skeletal muscle was significantly thinner in Hoxa5−/− and Hoxa5flox/flox; Olig2+/Cre specimens compared with controls and Hoxa5flox/flox; Dermo1+/Cre embryos (Fig. 7A-E). Both ventral and dorsal regions were equally affected, consistent with electron microscopy data. We also examined muscle fiber size by immunostaining for α-laminin, an extracellular matrix protein of basal lamina, which allows delineation of myofibers. Muscle fiber number per surface area was significantly augmented in Hoxa5−/− and Hoxa5flox/flox; Olig2+/Cre diaphragms, reflecting a decrease in myofiber size (Fig. 7F). Moreover, Hoxa5−/− and Hoxa5flox/flox; Olig2+/Cre diaphragms showed a decreased number of BrdU-positive nuclei compared with controls and Hoxa5flox/flox; Dermo1+/Cre specimens, indicating that decreased proliferation contributed to muscle atrophy (Fig. 7G).
Fig. 6.
Electron microscopy analysis of diaphragms from Hoxa5 mutants. (A-D′) Representative micrographs showing ultrastructure of diaphragms from E18.5 mutant embryos. Controls (A,A′) and Hoxa5flox/flox; Dermo1+/Cre (B,B′) specimens presented normal sarcomeres whereas Hoxa5flox/flox; Olig2+/Cre, (C,C′) and Hoxa5−/− (D,D′) diaphragms exhibited misaligned sarcomeres with a high proportion of non-contracted myofibers. Asterisks indicate damaged sarcomeric proteins and double arrowheads show wide I-bands. Lower panels show higher magnifications of the boxed areas above. Scale bars: 2 μm.
Fig. 7.
Diaphragm myogenesis and differentiation requires Hoxa5 expression in motor neurons. (A-D) α-Laminin immunofluorescence on E18.5 diaphragms delineates myofibers. (E-G) Thickness of the diaphragm (E), number of fibers per surface area (μm2) to assess myofiber size (F), and percentage of BrdU-positive cells (G) were measured on transverse sections of E18.5 specimens. (H) Runx1 expression was assessed by qRT-PCR on RNA from E18.5 diaphragms and was shown to be significantly increased in Hoxa5flox/flox; Olig2+/Cre and Hoxa5−/− specimens. (I,J) Expression of the myogenic regulators Pax3, Pax7, Myf5, Mrf4, Myog and Myod, and the growth factor Igf2 (I), and myosin heavy chain isoforms Myh7, Myh3, Myh8, Myh1, Myh2 and Myh4 (J) in E18.5 Hoxa5flox/flox, Hoxa5flox/flox; Olig2+/Cre and Hoxa5−/− diaphragms was assessed by qRT-PCR. Mean±s.e.m. are shown. *P<0.05, **P<0.01, ***P<0.001 (Student's t-test). Emb, embryonic; Neo, neonatal. Scale bar: 50 µm.
Muscle atrophy is associated with increased RUNX1 expression, a transcription factor induced in denervated muscle that helps to prevent myofibrillar disorganization and muscle wasting (Zhu et al., 1994; Wang et al., 2005). In Hoxa5−/− and Hoxa5flox/flox; Olig2+/Cre diaphragms, Runx1 expression was significantly increased in response to the incorrect innervation of the diaphragm (Fig. 7H). Thus, even if phrenic nerves reached the dorsal region of the diaphragm in these mutants, neuromuscular contacts appeared to be insufficient to properly support muscular development and growth, causing muscle atrophy. The decreased mechanical forces of the atrophic myofibers in turn likely caused a reduction in fetal breathing-like movements, contributing to lung hypoplasia and resulting in neonatal mortality.
Differentiation of diaphragm muscle fibers requires Hoxa5 expression in motor neurons
The defective sarcomeres, reduced diaphragm thickness and smaller cross-sectional area of the myofibers in Hoxa5−/− and Hoxa5flox/flox; Olig2+/Cre embryos raised the question of whether aberrant muscle differentiation contributes to the Hoxa5 phenotype. We thus analyzed expression of myogenic transcriptional regulators in these two mutants. Somitic myogenic precursors express Pax3 and Pax7 and the myogenic regulatory factor (MRF) Myf5 (Sambasivan and Tajbakhsh, 2007; Bentzinger et al., 2012). Expression of these three genes was significantly reduced in E18.5 Hoxa5−/− diaphragms, suggesting defective expansion, migration and/or maintenance of the resident myogenic progenitor population (Fig. 7I). Surprisingly, although Pax3 and Myf5 are genetically upstream of the MRF Myod (Myod1), its expression was significantly augmented in Hoxa5−/− specimens (Tajbakhsh et al., 1997). Abnormal expression of both Myod and Myf5 in Hoxa5−/− specimens might reflect aberrant progenitor fate decisions. In contrast, expression of the MRFs myogenin (Myog) and Mrf4 (Myf6), regulators of myocyte fusion and myotube formation, was unaffected (Fig. 7I). A negative-feedback loop exists between Myod and Igf2 in the diaphragm muscle, and double deletion of these genes causes diaphragm atrophy (Borensztein et al., 2013). Consistently, E18.5 Hoxa5−/− diaphragms also showed decreased Igf2 expression (Fig. 7I). Notably, none of these changes in myogenic regulatory expression was detected in Hoxa5flox/flox; Olig2+/Cre specimens, suggesting an additional role for Hoxa5 outside of motor neurons in diaphragm myogenesis.
Both Hoxa5flox/flox; Olig2+/Cre and Hoxa5−/− diaphragms showed a high proportion of non-contracted sarcomeres. We thus assessed myofiber type composition in these animals. During mouse embryonic diaphragm formation, two populations of fibers are produced in the first wave of myogenesis expressing either Myh3 and Myh7 or Myh3 and Myh8 myosin heavy chain isoforms, and giving rise to slow-twitch and fast-twitch muscle fibers, respectively. In the second wave, the developing myofibers only express Myh3 and Myh8 (Schiaffino and Reggiani, 2011). Myh7, Myh3 and Myh8 expression was significantly decreased in both Hoxa5flox/flox; Olig2+/Cre and Hoxa5−/− diaphragms (Fig. 7J). In contrast, expression of the adult heavy chain isoforms Myh1 and Myh2, which give rise to fast-twitch muscle fibers, presented an upward trend in these specimens. Together, these results suggested that innervation defects resulting from Hoxa5 deletion in phrenic motor neurons promoted the incorporation of fast-twitch fibers in diaphragm.
DISCUSSION
Hoxa5 is important for the formation of several organs and tissues, consistent with its tissue-specific expression in embryos and adults (Jeannotte et al., 2016). Hoxa5 is crucial for respiratory system development, and the null mutation disrupts formation of the trachea, lung and diaphragm causing respiratory failure and neonatal death (Aubin et al., 1997; Boucherat et al., 2013). HOXA5 expression is restricted to the mesenchymal layer of the respiratory tract and to the phrenic motor neurons, which are the sole source of diaphragm innervation. The lack of phenotype in Hoxa5flox/flox; Shh+/Cre specimens coupled to immunofluorescence data confirmed that Hoxa5 is not expressed and has no cell-autonomous function in the respiratory epithelium.
Although Hoxa5 is not required in lung epithelium, derivatives of both epithelium and mesenchyme are affected by the Hoxa5 mutation. Indeed, most lung phenotypes are epithelial, indicating that Hoxa5 acts primarily via mesenchymal-epithelial inductive signaling in this context. Hox5 compound mutants showed that Hox5 genes mainly control the canonical Wnt/Bmp4 signaling axis (Hrycaj et al., 2015). Here, analysis of Hoxa5 single mutants establishes the specific contribution of Hoxa5. Hoxa5 positively regulates the canonical Wnt pathway, and also controls expression of Fgf10, Shh and Bmp4, indicating a broad role in signal integration during lung morphogenesis. Hoxa5 action is also time dependent, as its influence on Wnt, Shh and Bmp4 lung expression decreases in older embryos. We previously reported that canonical Wnt activity was increased in adult Hoxa5−/− lungs, revealing a difference in how Hoxa5 controls the Wnt pathway in lung development compared with maintenance (Boucherat et al., 2012). Decreased Wnt activity in the developing lung might underlie the reduced proliferation and resulting lung hypoplasia in Hoxa5 mutants. Conversely, augmented Wnt activity in adults could explain the increased cell proliferation in lungs from Hoxa5 surviving mutants, allowing recovery of lung growth (Mandeville et al., 2006).
Hoxa5 positively regulates Fgf10 lung expression throughout embryogenesis. Hoxb5 also positively controls Fgf10 consistent with reduced lung branching in Hoxb5 mutants (Bellusci et al., 1997; Boucherat et al., 2013). Consequently, Fgf10 expression was also reduced in Hoxa5; Hoxb5 double mutants. The previously reported lack of Fgf10 expression change in E14.5 Hox5 triple mutants is therefore surprising (Hrycaj et al., 2015). One possibility could be negative regulation by the barely expressed Hoxc5 gene. Alternatively, variation in temporal Fgf10 expression might exist in Hox5 triple mutants. Finally, the presence of residual trachea tissue in lung samples from the triple mutants could explain the difference, as Hoxa5 does not regulate Fgf10 expression in upper airways.
Hoxa5 is the predominant Hox5 paralog to act in the lung airway smooth muscle program, where it regulates Pdgfra expression. Alveolar myofibroblasts are interstitial contractile cells responsible for elastin deposition and alveolar formation, two processes affected in Hoxa5−/− mutants (Mandeville et al., 2006). They are derived from embryonic lung mesenchyme, which expresses Pdgfra and is co-labeled with HOXA5 protein (Lindahl et al., 1997; Hamilton et al., 2003; Mandeville et al., 2006). This suggests a cell-autonomous function for Hoxa5 in alveolar myofibroblast progenitors (Fig. 8).
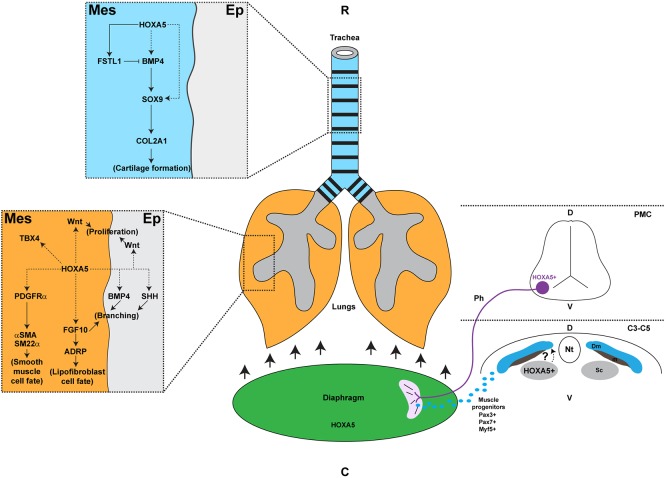
Fig. 8.
Hoxa5 distinct roles in the developing respiratory system. In trachea, HOXA5 expression in mesenchyme directly regulates Fstl1, an antagonist of BMP4 signaling. HOXA5 may also control Bmp4 and Sox9, which participate in tracheal cartilage formation. In lung, mesenchymal HOXA5 can activate Wnt ligand expression important for cell proliferation, Fgf10 involved in lung branching and lipofibroblast formation, and Pdgfra, essential for smooth muscle cell fate. HOXA5 may also positively control the epithelial expression of Bmp4 and Shh necessary for branching. Hoxa5 is also expressed in phrenic motor neurons, in the sclerotome of somites from the C3-C5 cervical region and in the diaphragm itself. Loss of Hoxa5 function in phrenic nerves impairs diaphragm innervation and musculature, which causes lung hypoplasia, respiratory distress and death at birth. The exact role of Hoxa5 in the diaphragm remains to be determined. C, caudal; C3-C5, cervical somites 3 to 5; D, dorsal; Dm, dermomyotome; Ep, epithelium; m, myotome; Mes, mesenchyme; Nt, neural tube; Ph, phrenic nerve; PMC, phrenic motor column; R, rostral; Sc, sclerotome; V, ventral.
Hoxa5 is also involved in lipofibroblast cell fate, as revealed by the reduced expression of peroxisome proliferator-activated receptor gamma (Pparg), a master regulator of adipogenesis, and adipose differentiation-related protein (Adrp; Plin2), a trafficking protein in lipofibroblasts (Fig. S7C, Fig. 8). Both genes are downstream of Fgf10 signaling during lipofibroblast formation (Al Alam et al., 2015). Moreover, the decreased expression of Tbx4, an early marker of lung mesenchymal cells, indicates that Hoxa5 may have a broad impact on lung mesenchymal cell fate, in addition to its instructive role on lung epithelium (Fig. S7C, Fig. 8) (Arora et al., 2012).
Tracheal cartilage malformations are fully penetrant in Hoxa5; Dermo1+/Cre mice, as in Hoxa5−/−, indicating that, as with lung development, it is the mesenchymal expression of Hoxa5 that regulates tracheal development. Incomplete Hoxa5 deletion by the Dermo1Cre, also establishes the requirement for a minimal level of Hoxa5 expression. Hoxb5 and Hoxc5 cannot rescue the Hoxa5 tracheal phenotype as they are not expressed in the developing upper airways (Boucherat et al., 2013). Therefore, Hoxa5 is the only Hox5 paralog gene involved in the tracheal developmental program.
In Hoxa5 mutants, tracheal cartilage rings form, thus mesenchymal cells become committed towards the chondrogenic lineage. SOX9 and HOXA5 are co-expressed in ventrolateral tracheal mesenchyme. Later, SOX9 becomes restricted to the condensing cartilage rings, whereas HOXA5 is present in ring cartilage and perichondrium and in surrounding mesenchyme (Fig. 1) (Arora et al., 2012). Hoxa5 mutant tracheas show both altered spatial expression of SOX9, and reduced Sox9 expression levels. Together, this suggests that Hoxa5 acts cell-autonomously to control mesenchymal Sox9 expression, which in turn permits the correct chondrogenesis of cartilage rings. This is consistent with the action of Hoxa5 on Sox9 expression during acromion formation and in chick somites, suggesting that it could be a conserved regulatory axis to direct cartilage development and morphology (Aubin et al., 2002; Chen et al., 2013).
Expression of Bmp4, and its target genes Id1 and Id2, was decreased in Hoxa5 mutant tracheas. BMP4 is a pro-chondrogenic signal that promotes chondrogenesis through Sox9 activation (Park et al., 2010b). Reduced BMP4 signaling might thus underlie the observed Sox9 downregulation. Moreover, Hoxa5 is co-expressed with Bmp4, making it a potential direct HOXA5 target (Li et al., 2008). Interestingly, expression of the BMP antagonist Fstl1 is also reduced in Hoxa5 mutant specimens, and Fstl1−/− mice present a malformed trachea with fewer cartilage rings and a disorganized epithelium reminiscent of the Hoxa5−/− tracheal phenotype (Geng et al., 2011). We previously showed that Fstl1 is a direct HOXA5 transcriptional target (Boucherat et al., 2013). The decreased expression of both Bmp4 and Fstl1 following the loss-of-Hoxa5 function appears to be contradictory. However, it underscores the complexity of the regulatory network in tracheal-bronchial chondrogenesis, which involves extensive cross-regulation between signaling pathways and transcriptional regulators by mechanisms that remain to be fully elucidated (Fig. 8).
Hoxa5flox/flox; Dermo1+/Cre and Hoxa5flox/−; Dermo1+/Cre adult mice were recovered at the expected Mendelian ratio indicating that tracheal malformations and compact lung architecture are not the cause of lethality of Hoxa5−/− newborns. In contrast, Hoxa5 deletion in phrenic motor neurons reproduced this lethality, demonstrating the crucial importance of Hoxa5 in diaphragm development and function. Only Hoxa5 mesenchymal expression appeared to be necessary for lung epithelial cell differentiation. However, both Hoxa5 mesenchymal and motor neuron mutations caused lung hypoplasia due to decreased proliferation. Thus, Hoxa5 has tissue-specific functions in mesenchyme and motor neurons that have both converging and distinct effects on respiratory development.
Hoxa5 expression in phrenic motor neurons is essential for diaphragm formation. Hoxa5flox/flox; Olig2+/Cre and Hoxa5−/− diaphragms were incorrectly innervated and showed abnormal musculature that evokes diaphragmatic eventration, a condition involving diaphragm relaxation that allows superior displacement of abdominal viscera into the thoracic cavity and impedes lung growth (Ackerman et al., 2012). Abnormal innervation was detected at E14.5, after the division of the phrenic nerve into the three primary branches. The failure of axons to reach the ventral part of the costal muscle, and the absence of recovery during diaphragm development, suggested that the defective innervation originates from axon guidance alterations rather than axonal degeneration and loss of synaptic contacts (Philippidou et al., 2012).
Profound alterations of the muscular component of diaphragm were also observed in Hoxa5−/− and Hoxa5flox/flox; Olig2+/Cre specimens, including thinner diaphragm, smaller myofibers and misaligned sarcomeres with damaged proteins, all characteristics of muscle atrophy (Wang et al., 2005; Borensztein et al., 2013). Increased Runx1 expression, a marker for damaged and regenerating muscle, further supported the notion of muscle atrophy and attempted recovery in Hoxa5 mutants (Umansky et al., 2015). Hoxa5−/− and Hoxa5flox/flox; Olig2+/Cre adult diaphragms presented an irregular distribution of costal muscles restricted to the ventral region (K.L.-T. and L.J., unpublished). This suggests that axon projections present in the dorsal diaphragm, although less abundant, were sufficient to generate synaptic contacts with muscle fibers and allow some muscle rescue.
Diaphragm muscles originate from myogenic precursor cells in the hypaxial dermomyotome of cervical somites C3 to C5, which express the myogenic transcriptional regulators Pax3, Pax7 and Myf5 (Bentzinger et al., 2012). Myf5−/− mice do not present diaphragm defects but Myf5; Myod double mutants, in which Mrf4 expression is compromised, lack diaphragm (Braun et al., 1992; Rudnicki et al., 1993; Kassar-Duchossoy et al., 2004). Splotch (Pax3−/−) mutants also lack diaphragm muscle, and that of Pax7−/− mice is notably thinner (Tremblay et al., 1998; Seale et al., 2000). Therefore, decreased expression of Pax3, Pax7 and Myf5 in Hoxa5−/− diaphragms might reflect a reduced contribution of myogenic progenitors to the diaphragm musculature.
Interestingly, Hoxa5flox/flox; Olig2+/Cre diaphragms do not show variation in Pax3, Pax7 or Myf5 expression. Thus, despite morphological similarity between both phenotypes, the diaphragm phenotype of Hoxa5−/− mutants might reflect Hoxa5 activity in cell types other than motor neurons. This could include the diaphragm connective tissue or somite-derived muscles. Hoxa5 is expressed in the somites from the cervical-thoracic transition domain C3 to T2. However, Hoxa5 somitic expression is confined to the lateral sclerotome, a source of cartilage and perichondrium, and no expression was detected in the dermomyotome of chicks or myotome of mice (Chen et al., 2013; J.H.M., unpublished). This suggests that the decreased expression of Pax3, Pax7 and Myf5 in Hoxa5−/− diaphragms is unlikely to result from a cell-autonomous action of Hoxa5 in myotome. However, we cannot exclude an indirect role for Hoxa5 somitic expression in the selective proliferation, migration and/or survival of myogenic precursors (Fig. 8).
Hoxa5 is also expressed in the developing diaphragm. However, no phenotype was detected in Hoxa5flox/flox; Dermo1+/Cre mutants, possibly owing to the incomplete activity of the Dermo1Cre allele in diaphragm. Cell lineage and co-labeling data indicated that Hoxa5 is not present in muscle cells and is likely expressed in the PPF lineage (Fig. 1, Fig. S1). Thus, other Cre deleter mouse lines will have to be tested to determine the role of Hoxa5 expression in the diaphragm.
Myod expression is positively regulated by both Pax3 and Myf5 (Tajbakhsh et al., 1997). However, Myod expression was increased in diaphragms from Hoxa5−/− mutants. One possible explanation is the decreased expression of Igf2 also observed in Hoxa5−/− diaphragms. Myod and Igf2 show reciprocal negative regulation in this context: Myod overexpression compensates for Igf2 downregulation and Igf2 overexpression compensates for Myod downregulation (Borensztein et al., 2013). The cause of reduced Igf2 expression in the diaphragm of Hoxa5−/− mutants remains unknown.
Myosin heavy chain fast isoform expression is augmented in Hoxa5−/− and Hoxa5flox/flox; Olig2+/Cre diaphragms. The fiber composition of a given muscle results from several factors including innervation, mechanical conditions and usage patterns. Moreover, when breathing rate increases, recruitment of fast muscle fibers is favored (Polla et al., 2004). Surviving Hoxa5−/− mice present respiratory adaptations including higher breathing frequency and augmented overall minute ventilation to circumvent morphological respiratory system defects (Kinkead et al., 2004). Together, our data suggest that they are compensatory mechanisms to maintain and adapt diaphragm muscle activity in order to allow the survival of Hoxa5 mutants.
In conclusion, characterization of Hoxa5 conditional mutants led us to establish that Hoxa5 regulates respiratory system development via several distinct and tissue-specific functions. These independent expression domains and functions are likely to reflect the stepwise origin and elaboration of respiratory structures in terrestrial vertebrates. For instance, the diaphragm and phrenic motor neurons that innervate it are an evolutionary novelty of mammals, and Hoxa5 was probably independently recruited for its various functions in the respiratory system as these structures arose (Jung and Dasen, 2015).
MATERIALS AND METHODS
Mouse lines, genotyping and tissue collection
The Hoxa5 null (Hoxa5tm1Rob), Hoxa5flox/flox (Hoxa5tm1Ljea), 14.5 kb Hoxa5-cre [Tg(Hoxa5-cre)447BLjea] and Olig2Cre [Olig2tm1(cre)Tmj] mouse lines were previously described (Jeannotte et al., 1993; Dessaud et al., 2007; Tabariès et al., 2007; Bérubé-Simard and Jeannotte, 2014). The Dermo1Cre [Twist2tm1(cre)Dor] line was obtained from Dr Ornitz (Washington University, St. Louis, MO, USA; Yu et al., 2003). The R26LacZ, R26mTmG and R26mT reporter lines [Gt(ROSA)26Sortm1Sor, Gt(ROSA)26Sortm4(ACTB−tdTomato,−EGFP)Luo and Gt(Rosa)26Sortm9(CAG−tdTomato)Hze)] and the ShhCre Washington University deleter strain [Shhtm1(EGFP/cre)Cjt] were purchased (Jackson Laboratory; Soriano, 1999; Harfe et al., 2004; Muzumdar et al., 2007; Madisen et al., 2010). As only individuals carrying the Hoxa5−/−, Hoxa5flox/flox; Dermo1+/Cre, Hoxa5flox/−; Dermo1+/Cre and Hoxa5flox/flox; Olig2+/Cre genotypes presented defects, all other genotypes were referred to as controls except when specified. Mouse lines were maintained in the 129/Sv background. Age of embryos was estimated by considering the morning of the day of the vaginal plug as E0.5. Experimental specimens were genotyped by PCR and Southern blot analyses. Trachea, lung and diaphragm from control and mutant embryos were collected at E12.5, E14.5, E18.5, P0 and P30. The wet lung and body weights were obtained by direct measurement. For RNA extraction, organs were snap-frozen in N2. Experiments were performed according to the guidelines of Canadian Council on Animal Care and approved by the Institutional Animal Care Committee.
Histology, IHC and immunofluorescence (IF) analyses
Specimens were processed for paraffin (4 µm) or frozen (5-10 µm) sections. Experiments were performed as described (Boucherat et al., 2017). Slides were counterstained with Nuclear Fast Red, Alcian Blue or Hematoxylin. For IF, nuclei were visualized by DAPI staining. The Cyanine 3 Tyramide Signal Amplification Kit (Perkin Elmer) was used at 1/50 dilution for HOXA5 IF detection. Antibodies are listed in Table S1.
β-Galactosidase staining
Detection of β-galactosidase activity in frozen sections (10 µm) from E12.5 embryos was performed as described (Bérubé-Simard and Jeannotte, 2014).
Proliferation assay
Pregnant females were injected intraperitoneally with 100 µg BrdU/g body weight and embryos were collected 1 h later. Proliferation index corresponds to the number of BrdU-immunoreactive cells divided by total cell number for each section analyzed. Four to eight random fields were taken for an average number of 200 diaphragm and 750 lung cells per field, from three to five embryos per genotype.
Electron microscopy
Diaphragms from E18.5 embryos were divided into dorsal-left, dorsal-right, ventral-left and ventral-right regions, fixed in 0.1 M sodium cacodylate, pH7.3, containing 2.5% glutaraldehyde for 24 h, washed in sodium cacodylate buffer, post-fixed in 1% OsO4, dehydrated in ethanol and then propylene oxide, embedded in Epon, and sectioned at 60-80 nm. Sections were stained in 0.4% lead citrate for 8 min and in 3% uranyl acetate solution for 5 min. Pictures were taken with a transmission electron microscope JEOL, JEM1230 (Tokyo, Japan) used at 80 kV and equipped with two CCD cameras: (1) wide-field Gatan Dual Vision and (2) high-resolution Gatan Ultrascan 1000XP.
Alcian Blue cartilage staining
Dissected respiratory tracts from E18.5 embryos were stained overnight at 37°C in 0.015% Alcian Blue, 20% acetic acid prepared in 95% ethanol. Specimens were processed 30 min in 1% KOH for better visualization of cartilage rings.
Morphometric measurements
ImageJ software was used to calculate (1) the tracheal length between the cricoid cartilage and the carina, (2) the trachea external diameter along the most linear portion of trachea, and (3) the number of diaphragm muscle fibers/area (μm2) at eight dorso-ventral locations in the right costal muscle. Using the Leica SCN 400 F SlideScanner and SlidePath Gateway Software, we measured the tracheal luminal surface, and the diaphragm thickness at eight dorso-ventral locations in the right costal muscle. Three to eleven specimens were used for each genotype tested.
qRT-PCR assays
Total RNA was isolated from trachea, lung and diaphragm of individuals. qRT-PCR experiments were performed as described (Boucherat et al., 2012). Three to eight specimens were used for each genotype tested. Primer sequences are listed in Table S2.
Statistical analyses
Student's t-test was performed for comparative studies. A significance level below 5% (P<0.05) was considered statistically significant. Values are expressed as mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001.
Acknowledgements
We thank Drs Jean Charron and Luisa Dandolo for comments, Dr David Ornitz for Dermo1Cre mice, Drs Normand Marceau and Gurmukh Singh for antibodies, and Richard Janvier for electron microscopy analysis.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: K.L.-T., O.B., L.J.; Methodology: K.L.-T., N.H., O.B., F.-H.J., P.P., L.J.; Validation: K.L.-T., N.H., O.B., F.-H.J., P.P.; Formal analysis: K.L.-T., N.H., O.B., F.-H.J., P.P., L.J.; Resources: J.S.D., J.H.M.; Writing - original draft: K.L.-T., O.B., J.H.M., L.J.; Writing - review & editing: K.L.-T., O.B., P.P., J.H.M., L.J.; Supervision: L.J.; Project administration: L.J.; Funding acquisition: L.J.
Funding
This work was supported by grants from Canadian Institutes of Health Research (MOP-15139 to L.J.) and Natural Sciences and Engineering Research Council of Canada (NSERC) (194559 to L.J.) and by a NSERC summer studentship (to F.-H.J.).
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.152686.supplemental
References
- Ackerman K. G., Vargas S. O., Wilson J. A., Jennings R. W., Kozakewich H. P. W. and Pober B. R. (2012). Congenital diaphragmatic defects: proposal for a new classification based on observations in 234 patients. Pediatr. Dev. Pathol. 15, 265-274. 10.2350/11-05-1041-OA.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Alam D., El Agha E., Sakurai R., Kheirollahi V., Moiseenko A., Danopoulos S., Shrestha A., Schmoldt C., Quantius J., Herold S. et al. (2015). Evidence for the involvement of fibroblast growth factor 10 in lipofibroblast formation during embryonic lung development. Development 142, 4139-4150. 10.1242/dev.109173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan D. W. and Greer J. J. (1997). Embryogenesis of the phrenic nerve and diaphragm in the fetal rat. J. Comp. Neurol. 382, 459-468. [DOI] [PubMed] [Google Scholar]
- Arora R., Metzger R. J. and Papaioannou V. E. (2012). Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLoS Genet. 8, e1002866 10.1371/journal.pgen.1002866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin J., Lemieux M., Tremblay M., Bérard J. and Jeannotte L. (1997). Early postnatal lethality in Hoxa-5 mutant mice is attributable to respiratory tract Defects. Dev. Biol. 192, 432-445. 10.1006/dbio.1997.8746 [DOI] [PubMed] [Google Scholar]
- Aubin J., Lemieux M., Moreau J., Lapointe J. and Jeannotte L. (2002). Cooperation of Hoxa5 and Pax1 genes during formation of the pectoral girdle. Dev. Biol. 244, 96-113. 10.1006/dbio.2002.0596 [DOI] [PubMed] [Google Scholar]
- Bellusci S., Grindley J., Emoto H., Itoh N. and Hogan B. L. (1997). Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 124, 4867-4878. [DOI] [PubMed] [Google Scholar]
- Bentzinger C. F., Wang Y. X. and Rudnicki M. A. (2012). Building muscle: molecular regulation of myogenesis. Cold Spring Harb. Perspect Biol. 4, pii:a008342 10.1101/cshperspect.a008342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bérubé-Simard F.-A. and Jeannotte L. (2014). Hoxa5/Cre transgenic mice: Novel tools for regional deletion along the anterior-posterior axis. genesis 52, 149-156. 10.1002/dvg.22733 [DOI] [PubMed] [Google Scholar]
- Borensztein M., Monnier P., Louault Y., Ripoche M.-A., Tiret L., Yao Z., Tapscott S. J., Forné T., Montarras D. and Dandolo L. (2013). Myod and H19-Igf2 locus interactions are required for diaphragm formation in the mouse. Development 140, 1231-1239. 10.1242/dev.084665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucherat O., Chakir J. and Jeannotte L. (2012). The loss of Hoxa5 function promotes Notch-dependent goblet cell metaplasia in lung airways. Biol. Open 1, 677-691. 10.1242/bio.20121701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucherat O., Montaron S., Bérubé-Simard F.-A., Aubin J., Philippidou P., Wellik D. M., Dasen J. S. and Jeannotte L. (2013). Partial functional redundancy between Hoxa5 and Hoxb5 paralog genes during lung morphogenesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 304, L817-L830. 10.1152/ajplung.00006.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucherat O., Nadeau V., Bérubé-Simard F.-A., Charron J. and Jeannotte L. (2014). Crucial requirement of ERK/MAPK signaling in respiratory tract development. Development 141, 3197-3211. 10.1242/dev.110254 [DOI] [PubMed] [Google Scholar]
- Boucherat O., Landry-Truchon K., Aoidi R., Houde N., Nadeau V., Charron J. and Jeannotte L. (2017). Lung development requires an active ERK/MAPK pathway in the lung mesenchyme. Dev. Dyn. 246, 72-82. 10.1002/dvdy.24464 [DOI] [PubMed] [Google Scholar]
- Braun T., Rudnicki M. A., Arnold H.-H. and Jaenisch R. (1992). Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell 71, 369-382. 10.1016/0092-8674(92)90507-9 [DOI] [PubMed] [Google Scholar]
- Chang D. R., Martinez Alanis D., Miller R. K., Ji H., Akiyama H., McCrea P. D. and Chen J. (2013). Lung epithelial branching program antagonizes alveolar differentiation. Proc. Natl. Acad. Sci. USA 110, 18042-18051. 10.1073/pnas.1311760110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. W., Zahid S., Shilts M. H., Weaver S. J., Leskowitz R. M., Habbsa S., Aronowitz D., Rokins K. P., Chang Y., Pinnella Z. et al. (2013). Hoxa-5 acts in segmented somites to regulate cervical vertebral morphology. Mech. Dev. 130, 226-240. 10.1016/j.mod.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Coulombe Y., Lemieux M., Moreau J., Aubin J., Joksimovic M., Bérubé-Simard F.-A., Tabariès S., Boucherat O., Guillou F., Larochelle C. et al. (2010). Multiple promoters and alternative splicing: Hoxa5 transcriptional complexity in the mouse embryo. PLoS ONE 5, e10600 10.1371/journal.pone.0010600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessaud E., Yang L. L., Hill K., Cox B., Ulloa F., Ribeiro A., Mynett A., Novitch B. G. and Briscoe J. (2007). Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature 450, 717-720. 10.1038/nature06347 [DOI] [PubMed] [Google Scholar]
- Elluru R. G. and Whitsett J. A. (2004). Potential role of Sox9 in patterning tracheal cartilage ring formation in an embryonic mouse model. Arch. Otolaryngol. Head Neck Surg. 130, 732-736. 10.1001/archotol.130.6.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y., Dong Y., Yu M., Zhang L., Yan X., Sun J., Qio L., Geng H., Nakajima M., Furuichi T. et al. (2011). Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc. Natl. Acad. Sci. USA 108, 7058-7063. 10.1073/pnas.1007293108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss A. M., Tian Y., Cheng L., Yang J., Zhou D., Cohen E. D. and Morrisey E. E. (2011). Wnt2 signaling is necessary and sufficient to activate the airway smooth muscle program in the lung by regulating myocardin/Mrtf-B and Fgf10 expression. Dev. Biol. 356, 541-552. 10.1016/j.ydbio.2011.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer J. J., Allan D. W., Martin-Caraballo M. and Lemke R. P. (1999). An overview of phrenic nerve and diaphragm muscle development in the perinatal rat. J. Appl. Physiol. 86, 779-786. 10.1063/1.370804 [DOI] [PubMed] [Google Scholar]
- Hamilton T. G., Klinghoffer R. A., Corrin P. D. and Soriano P. (2003). Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol. Cell. Biol. 11, 4013-4025. 10.1128/MCB.23.11.4013-4025.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe B. D., Scherz P. J., Nissim S., Tian H., McMahon A. P. and Tabin C. J. (2004). Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517-528. 10.1016/j.cell.2004.07.024 [DOI] [PubMed] [Google Scholar]
- Herriges J. C., Yi L., Hines E. A., Harvey J. F., Xu G., Gray P. A., Ma Q. and Sun X. (2012). Genome-scale study of transcription factor expression in the branching mouse lung. Dev. Dyn. 241, 1432-1453. 10.1002/dvdy.23823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B. L., Barkauskas C. E., Chapman H. A., Epstein J. A., Jain R., Hsia C. C., Niklason L., Calle E., Le A., Randell S. H. et al. (2014). Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cells 15, 123-138. 10.1016/j.stem.2014.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrycaj S. M., Dye B. R., Baker N. C., Larsen B. M., Burke A. C., Spence J. R. and Wellik D. M. (2015). Hox5 genes regulate the Wnt2/2b-Bmp4-signaling axis duringl development. Cell Rep. 12, 903-912. 10.1016/j.celrep.2015.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay P. Y., Bielinska M., Erlich J. M., Mannisto S., Pu W. T., Heikinheimo M. and Wilson D. B. (2007). Impaired mesenchymal cell function in Gata4 mutant mice leads to diaphragmatic hernias and primary lung defects. Dev. Biol. 301, 602-614. 10.1016/j.ydbio.2006.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannotte L., Lemieux M., Charron J., Poirier F. and Robertson E. J. (1993). Specification of axial identity in the mouse: role of the Hoxa-5 (Hox1. 3) gene. Genes Dev. 7, 2085-2096. 10.1101/gad.7.11.2085 [DOI] [PubMed] [Google Scholar]
- Jeannotte L., Gotti F. and Landry-Truchon K. (2016). Hoxa5: a key player in development and disease. J. Dev. Biol. 4, 13 10.3390/jdb4020013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H. and Dasen J. S. (2015). Evolution of patterning systems and circuit elements for locomotion. Dev. Cell 32, 408-422. 10.1016/j.devcel.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassar-Duchossoy L., Gayraud-Morel B., Gomès D., Rocancourt D., Buckingham M., Shinin V. and Tajbakhsh S. (2004). Mrf4 determines skeletal muscle identity in Myf5:Myod double-mutant mice. Nature 431, 466-471. 10.1038/nature02876 [DOI] [PubMed] [Google Scholar]
- Kinkead R., Leblanc M., Gulemetova R., Lalancette-Hébert M., Lemieux M., Mandeville I. and Jeannotte L. (2004). Respiratory adaptations to lung morphological defects in adult mice lacking Hoxa5 gene function. Ped. Res. 56, 553-562. 10.1203/01.PDR.0000139427.26083.3D [DOI] [PubMed] [Google Scholar]
- Kotecha S. (2000). Lung growth for beginners. Paed. Respir. Rev. 1, 308-313. 10.1053/prrv.2000.0069 [DOI] [PubMed] [Google Scholar]
- Lefebvre V., Huang W., Harley V. R., Goodfellow P. N. and de Combrugghe B. (1997). SOX9 is a potent activator of the chondrocyte-specific enhancer of the Proα1(II) collagen gene. Mol. Cell. Biol. 17, 2336-2346. 10.1128/MCB.17.4.2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V., Li P. and de Combrugghe B. (1998). A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 17, 5718-5733. 10.1093/emboj/17.19.5718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Gordon J., Manley N. R., Litingtung Y. and Chiang C. (2008). Bmp4 is required for tracheal formation: A novel mouse model for tracheal agenesis. Dev. Biol. 322, 145-155. 10.1016/j.ydbio.2008.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggins G. C., Vilos G. A., Campos G. A., Kitterman J. A. and Lee C. H. (1981). The effect of spinal cord transection on lung development in fetal sheep. J. Dev. Physiol. 3, 267-274. [PubMed] [Google Scholar]
- Lindahl P., Karlsson L., Hellström M., Gebre-Medhin S., Willetts K., Heath J. K. and Betsholtz C. (1997). Alveogenesis failure in PDGF-A deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development 124, 3943-3953. [DOI] [PubMed] [Google Scholar]
- Madisen L., Zwingman T. A., Sunkin S. M., Oh S. W., Zarwala H. A., Gu H., Ng L. L., Palmiter R. D., Hawrylycz M. J., Jones A. R. et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133-140. 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeville I., Aubin J., LeBlanc M., Lalancette-Hébert M., Janelle M.-F., Tremblay G. M. and Jeannotte L. (2006). Impact of the loss of Hoxa5 function on lung alveogenesis. Am. J. Pathol. 169, 1312-1327. 10.2353/ajpath.2006.051333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W. and Krumlauf R. (1992). Homeobox genes and axial patterning. Cell 68, 283-302. 10.1016/0092-8674(92)90471-N [DOI] [PubMed] [Google Scholar]
- Merrell A. J. and Kardon G. (2013). Development of the diaphragm–a skeletal muscle essential for mammalian respiration. FEBS J. 280, 4026-4035. 10.1111/febs.12274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell A. J., Ellis B. J., Fox Z. D., Lawson J. A., Weiss J. A. and Kardon G. (2015). Muscle connective tissue controls development of the diaphragm and is a source of congenital diaphragmatic hernias. Nat. Genet. 47, 496-504. 10.1038/ng.3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. F., Cohen E. D., Baggs J. E., Lu M. M., Hogenesch J. B. and Morrisey E. E. (2012). Wnt ligands signal in a cooperative manner to promote foregut organogenesis. Proc. Natl. Acad. Sci. USA 109, 15348-15353. 10.1073/pnas.1201583109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey E. E. and Hogan B. L. M. (2010). Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev. Cell 18, 8-23. 10.1016/j.devcel.2009.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar M. D., Tasic B., Miyamichi K., Li L. and Luo L. (2007). A global double-fluorescent Cre reporter mouse. Genesis 45, 593-605. 10.1002/dvg.20335 [DOI] [PubMed] [Google Scholar]
- Park G.-H., Maeno-Hikichi Y., Awano T., Landmesser L. T. and Monani U. R. (2010a). Reduced survival of motor neuron (SMN) protein in motor neuronal progenitors functions cell autonomously to cause spinal muscular atrophy in model mice expressing the human centromeric (SMN2) gene. J. Neuro. 30, 12005-12019. 10.1523/JNEUROSCI.2208-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Zhang J. J. R., Moro A., Kushida M., Wegner M. and Kim P. C. W. (2010b). Regulation of Sox9 by Sonic Hedgehog (Shh) is essential for patterning and formation of tracheal cartilage. Dev. Dyn. 239, 514-526. 10.1002/dvdy.22192 [DOI] [PubMed] [Google Scholar]
- Philippidou P., Walsh C. M., Aubin J., Jeannotte L. and Dasen J. S. (2012). Sustained Hox5 gene activity is required for respiratory motor neuron development. Nat. Neurosci. 15, 1636-1644. 10.1038/nn.3242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polla B., D'Antona G., Bottinelli R. and Reggiani C. (2004). Respiratory muscle fibres: specialisation and plasticity. Thorax 59, 808-817. 10.1136/thx.2003.009894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki M. A., Schnegelsberg P. N. J., Stead R. H., Braun T., Arnold H.-H. and Jaenisch R. (1993). MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 75, 1351-1359. 10.1016/0092-8674(93)90621-V [DOI] [PubMed] [Google Scholar]
- Sala F. G., Del Moral P.-M., Tiozzo C., Alam D. A., Warburton D., Grikscheit T., Veltmaat J. M. and Bellusci S. (2011). FGF10 controls the patterning of the tracheal cartilage rings via Shh. Development 138, 273-282. 10.1242/dev.051680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambasivan R. and Tajbakhsh S. (2007). Skeletal muscle stem cell birth and properties. Sem. Cell. Dev. Biol. 18, 870-882. 10.1016/j.semcdb.2007.09.013 [DOI] [PubMed] [Google Scholar]
- Schiaffino S. and Reggiani C. (2011). Fiber types in mammalian skeletal muscles. Physiol. Rev. 91, 1447-1531. 10.1152/physrev.00031.2010 [DOI] [PubMed] [Google Scholar]
- Seale P., Sabourin L. A., Girgis-Gabardo A., Mansouri A., Gruss P. and Rudnicki M. A. (2000). Pax7 is required for the specification of myogenic satellite cells. Cell 102, 777-786. 10.1016/S0092-8674(00)00066-0 [DOI] [PubMed] [Google Scholar]
- Shu W., Guttentag S., Wang Z., Andl T., Ballard P., Lu M. M., Piccolo S., Birchmeier W., Whitsett J. A., Millar S. E.. et al. (2005). Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev. Biol. 283, 226-239. 10.1016/j.ydbio.2005.04.014 [DOI] [PubMed] [Google Scholar]
- Soriano P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genet. 21, 70-71. 10.1038/5007 [DOI] [PubMed] [Google Scholar]
- Tabariès S., Lemieux M., Aubin J. and Jeannotte L. (2007). Comparative analysis of Hoxa5 allelic series. genesis 45, 218-228. 10.1002/dvg.20292 [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S., Rocancourt D., Cossu G. and Buckingham M. (1997). Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell 89, 127-138. 10.1016/S0092-8674(00)80189-0 [DOI] [PubMed] [Google Scholar]
- Tian Y., Zhang Y., Hurd L., Hannenhalli S., Liu F., Lu M. M. and Morrisey E. E. (2011). Regulation of lung endoderm progenitor cell behavior by miR302/367. Development 138, 1235-1245. 10.1242/dev.061762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay P., Dietrich S., Mericskay M., Schubert F. R., Li Z. and Paulin D. (1998). A crucial role for Pax3 in the development of the hypaxial musculature and the long-range migration of muscle precursors. Dev. Biol. 203, 49-61. 10.1006/dbio.1998.9041 [DOI] [PubMed] [Google Scholar]
- Turcatel G., Rubin N., Menke D. B., Martin G., Shi W. and Warburton D. (2013). Lung mesenchymal expression of Sox9 plays a critical role in tracheal development. BMC Biol. 11, 117 10.1186/1741-7007-11-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umansky K. B., Gruenbaum-Cohen Y., Tsoory M., Feldmesser E., Goldenberg D., Brenner O. and Groner Y. (2015). Runx1 transcription factor is required for myoblasts proliferation during muscle regeneration. PLoS Genet. 11, e1005457 10.1371/journal.pgen.1005457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Blagden C., Fan J., Nowak S. J., Taniuchi I., Littman D. R. and Burden S. J. (2005). Runx1 prevents wasting, myofibrillar disorganization, and autophagy of skeletal muscle. Genes Dev. 19, 1715-1722. 10.1101/gad.1318305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigglesworth J. S. and Desai R. (1979). Effects on lung growth of cervical cord section in the rabbit fetus. Early Human Dev. 3, 51-65. 10.1016/0378-3782(79)90020-3 [DOI] [PubMed] [Google Scholar]
- Yu K., Xu J., Liu Z., Sosic D., Shao J., Olson E. N., Towler D. A. and Ornitz D. M. (2003). Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development 130, 3063-3074. 10.1242/dev.00491 [DOI] [PubMed] [Google Scholar]
- Zhu X., Yeadon J. E. and Burden S. J. (1994). AML1 is expressed in skeletal muscle and is regulated by innervation. Mol. Cell. Biol. 14, 8051-8057. 10.1128/MCB.14.12.8051 [DOI] [PMC free article] [PubMed] [Google Scholar]