Fig. 4.
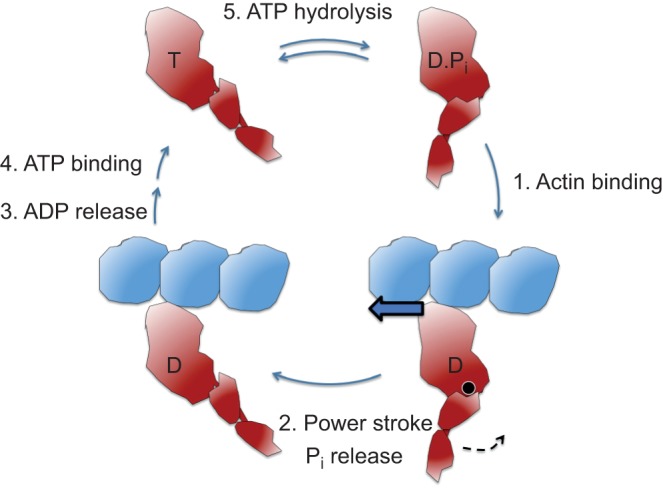
Schematic figure of the key steps of the actin-activated myosin chemomechanical cycle. The total cycle involves five key steps. The pre-stroke states of S1 are shown on the right and the post-stroke states on the left. Step 1: The pre-stroke S1 with bound ADP (D) and phosphate (Pi) undergoes a weak to strong transition in binding to actin. This is the rate-limiting step in the cycle and determines the total cycle time (tc), which is 1/kcat, where kcat is the turnover number, or the number of substrate molecules each enzyme site converts to product per unit time. Step 2: While tightly bound to actin, the lever arm swings about its fulcrum point (black circle) to the right to its post-stroke position (black arrow), moving the actin filament to the left (bold blue arrow) with respect to the myosin thick filament. Step 3: ADP release frees the active site for binding of ATP (T). This step is related to the velocity of the actin filament sliding as the filament cannot move any faster than the myosin heads can let go. Step 4: ATP binding weakens the interaction of the S1 to actin. Although the ATP-bound heads still maintain a weak affinity for the actin, this state is typically drawn as dissociated from the actin filament. Step 5: ATP hydrolysis results in cocking of the head into the pre-stroke state (Shih et al., 2000).
