Abstract
Emphysematous gastritis is a rare and lethal gastrointestinal emergency if not promptly identified and managed. In rare cases, emphysematous gastritis is associated with Sarcina ventriculi infection, usually in patients with delayed gastric emptying. Here we report a lethal case of S. ventriculi-associated emphysematous gastritis in the absence of delayed gastric emptying in which the diagnosis was confirmed postmortem. This case provides an opportunity to review the clinical presentation, pathophysiology, and management of emphysematous gastritis so that the condition can be promptly diagnosed and managed to prevent significant morbidity and mortality.
Keywords: Emphysematous gastritis, Sarcina ventriculi, Gastric emphysema
Introduction
Emphysematous gastritis is a rare and usually lethal gastrointestinal emergency, with an estimated mortality rate approaching 60% [1]. The condition is associated with chronic alcohol consumption, recent abdominal surgery, leukemia, lymphoma, renal failure, diabetes mellitus, gastric wall corrosion from acid or alkali poisoning, acute pancreatitis, disseminated strongyloidiasis, adenocarcinoma, phytobezoar consumption, and long-term treatment with steroids or antibiotics [2]. Infections are an important but less common cause of emphysematous gastritis and are generally present in patients with predisposing conditions that delay gastric emptying. The infections most frequently associated with emphysematous gastritis include the gas-producing bacteria Clostridium perfringens, Enterobacter spp., Klebsiella pneumoniae, Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, Pseudomonas aeruginosa, Proteus spp., and sometimes fungi such as Mucormycosis spp. and Candida glabrata, krusei, and albicans [3]. Emphysematous gastritis can also be caused by Sarcina ventriculi, a Gram-negative, gas-producing bacterium that has very rarely been described in association with emphysematous gastritis in the absence of delayed gastric emptying.
Here we present a rare but fatal case of emphysematous gastritis confirmed after postmortem review in a patient without diabetic gastroparesis but with a preceding S. ventriculi infection. Since this condition is uncommon and lethal in most cases, we use this opportunity to review its clinical presentation, pathophysiology, and management so that gastroenterologists and other clinicians can quickly identify the condition, promptly intervene, and prevent significant morbidity and mortality.
Case Description
An 86-year-old woman with a history of hypertension and type II diabetes mellitus presented to the emergency department with a 3-day history of diffuse stabbing abdominal pain, nausea, vomiting, loss of appetite, and diarrhea described as black by her family. At presentation, she was afebrile and normotensive but appeared confused. There was generalized abdominal tenderness without guarding, rigidity, or rebound tenderness. She had a white cell count of 33 × 103 cells/μL (normal 4.8–10.8 × 103), serum creatinine 1.76 mg/dL (normal 0.8–2 mg/dL), high anion gap metabolic acidosis with serum bicarbonate 11 mmol/L (normal 24–31 mmol/L), an anion gap of 33 mmol/L (normal 5–15 mmol/L), beta-hydroxybutyrate 2.71 mmol/L (normal 0.02–0.28 mmol/L), and a lactic acid of 4.6 mmol/L (normal 0.5–2.2 mmol/L). An abdominal CT scan revealed air in the stomach wall and portal vein, air distending the stomach, and extensive prominent small bowel looping (Fig. 1). Intravenous fluids, vancomycin, piperacillin-tazobactam, and pantoprazole were started for the presumed diagnosis of emphysematous gastritis. The patient then had massive hematemesis, after which she rapidly became hemodynamically unstable. Despite resuscitation efforts, the patient died. A postmortem review confirmed the underlying diagnosis of emphysematous gastritis (Fig. 2) in the presence of bacterial overgrowth by S. ventriculi (Fig. 3a, b).
Fig. 1.
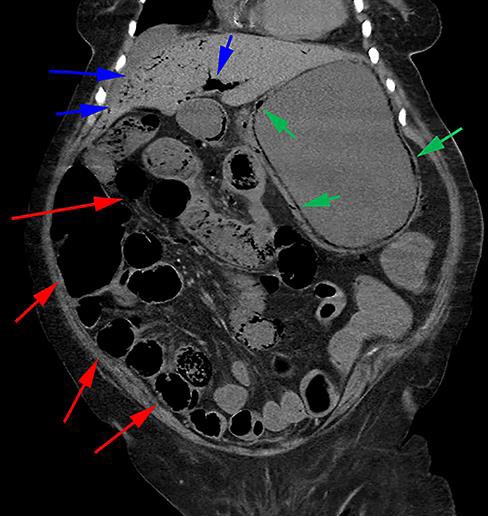
Abdominal CT scan revealing marked air within the gastric wall (green arrows), extensive prominent looping of the small bowel (red arrows), and air within the portal vein (blue arrows).
Fig. 2.

Gross specimen of the stomach retrieved from autopsy showing areas of hyperemia and necrosis suggestive of emphysematous gastritis.
Fig. 3.

Photomicrographs of the stomach stained with hematoxylin and eosin. a 4× magnification showing air sacs within the stomach suggestive of emphysematous gastritis. b 40× magnification of the stomach specimen demonstrating the characteristic features of bacterial overgrowth by S. ventriculi, namely basophilic, cuboid-shaped organisms in a tetrad formation.
Discussion
S. ventriculi, a Gram-negative obligate anaerobe that ferments carbohydrates to produce carbon dioxide and ethanol as by-products, was first reported as an animal infection and also in the feces of healthy humans consuming a predominantly vegetarian diet [4]. However, since these initial reports, there have been increasing reports of Sarcina spp. in association with peritonitis after gastrointestinal viscous perforation and, rarely, emphysematous gastritis [5, 6].
Emphysematous gastritis is very rare, so its exact pathophysiology remains unclear. However, it is thought that a preexisting gastric ulcer serves as a nidus for bacterial infection, overgrowth, and penetration into the gastric wall [6]. After penetration, the organisms produce gas. In the case of S. ventriculi, bacterial overgrowth is promoted by underlying conditions associated with delayed gastric emptying such as diabetic gastroparesis, pyloric stenosis, gastric surgery, slipped gastric banding, scarring, or obstructing masses, because gastric content retention and decreased gastric outflow provide time for the organisms to grow and penetrate the gastric wall [7]. Furthermore, overgrowth of Sarcina spp. is promoted by this organism's ability to survive and grow even at pH 1, essentially evading the natural defense mechanism of the stomach's acidic environment [8, 9].
Identifying the symptoms of emphysematous gastritis can be challenging and hence catastrophic when missed. The most frequent symptoms include abdominal pain, nausea, vomiting, and, in some cases, fever and chills. These symptoms can cause clinical confusion since they are nonspecific and there is considerable overlap with the symptoms of gastroenteritis. However, the presence of hematemesis, melena, leukocytosis, portal vein gas, lactic acidosis, and hemodynamic instability or hypotension arouse suspicion and support the diagnosis of emphysematous gastritis [10, 11]. Diagnostic testing for emphysematous gastritis involves plain abdominal radiographs or abdominal CT, which may show air within the gastric wall and portal vein [2]. Clinically, gas within the stomach wall can be categorized into two groups: emphysematous gastritis and gastric emphysema, a relatively benign condition caused by air entering the gastric wall, usually following trauma to the gastric mucosa. This distinction is critical, since patients with gastric emphysema are generally asymptomatic and the air in the stomach wall is generally considered benign, in contrast to emphysematous gastritis, which is never benign [10, 11].
The management of emphysematous gastritis is challenging because patients can quickly deteriorate and become hemodynamically unstable, as seen in this case. The most ominous sign in these patients is the radiographic finding of portal vein gas caused either by intraluminal air or air from the bacterium [12, 13]. This sign generally represents the worst prognostic marker in these patients, with an estimated mortality exceeding 75% when present [13]. Despite a lack of consensus guidelines for the treatment of emphysematous gastritis, a therapeutic approach based on disease severity has been devised in a few case reports [10, 14, 15]. Prompt intervention with intravenous antibiotics, analgesia, total parenteral nutrition, and proton pump inhibitors should be attempted. There are some reports in the literature of gastrectomy as a potential treatment option; however, patient age and comorbidities should be weighed accordingly in discussions regarding the risks and benefits of invasive procedures because of the high mortality rate associated with this condition [16]. Though the role of surgery during the acute phase remains unclear, indications for emergency surgery include clinical deterioration despite medical treatment, involvement of a large portion of or the entire stomach, and gastric infarction or perforation [16, 17]. Other complications such as development of strictures may occur in approximately 25% of patients as late findings during healing and are considered absolute indications for surgery [18].
In summary, we report a case of emphysematous gastritis in association with S. ventriculi in the absence of the most common risk factors including delayed gastric emptying. The underlying etiology was unclear. Although treatment was started promptly and death was probably inevitable, this case serves as a reminder to gastroenterologists and other clinicians that a high degree of suspicion for emphysematous gastritis should be maintained when patients present with abdominal pain, hematemesis, and/or melena. Imaging that shows gas within the wall of the stomach and portal vein must be acted on immediately so that appropriate interventions can be initiated to prevent mortality. Clinicians should also be aware that S. ventriculi can present in patients in the absence of delayed gastric emptying. Due to the rarity of this condition and the lack of clear consensus guidelines, future reports may be useful to identify cases that may benefit from less invasive therapies such as endoscopy to spare patients from surgery.
Statement of Ethics
This study was completed in compliance with guidelines for human studies and is ethically in accordance with the World Medical Association Declaration of Helsinki. The patient's next of kin gave written informed consent to publish this case and images.
Disclosure Statement
The author has no conflicts of interest to declare. He declares no relevant funding sources.
Author Contributions
Dr. K. Singh wrote and prepared this paper.
Acknowledgments
The author thanks Dr. Youyuan Xu for performing the postmortem examination and preparing the photographs of the gross anatomy and the photomicrographs included in this paper.
References
- 1.Hadas-Halpren I, Hiller N, Guberman D. Emphysematous gastritis secondary to ingestion of large amounts of Coca Cola. Am J Gastroenterol. 1993 Jan;88((1)):127–9. [PubMed] [Google Scholar]
- 2.Paul M, John S, Menon MC, Golewale NH, Weiss SL, Murthy UK. Successful medical management of emphysematous gastritis with concomitant portal venous air: a case report. J Med Case Reports. 2010 May;4((1)):140. doi: 10.1186/1752-1947-4-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Mook WN, van der Geest S, Goessens ML, Schoon EJ, Ramsay G. Gas within the wall of the stomach due to emphysematous gastritis: case report and review. Eur J Gastroenterol Hepatol. 2002 Oct;14((10)):1155–60. doi: 10.1097/00042737-200210000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Crowther JS. Sarcina ventriculi in human faeces. J Med Microbiol. 1971 Aug;4((3)):343–50. doi: 10.1099/00222615-4-3-343. [DOI] [PubMed] [Google Scholar]
- 5.Tolentino LF, Kallichanda N, Javier B, Yoshimori R, French SW. A Case Report of Gastric Perforation and Peritonitis Associated With Opportunistic Infection by Sarcina ventriculi. Lab Med. 2003;34((7)):535–7. [Google Scholar]
- 6.Lam-Himlin D, Tsiatis AC, Montgomery E, Pai RK, Brown JA, Razavi M, et al. Sarcina organisms in the gastrointestinal tract: a clinicopathologic and molecular study. Am J Surg Pathol. 2011 Nov;35((11)):1700–5. doi: 10.1097/PAS.0b013e31822911e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, Gopal P. Sarcina ventriculi in a Patient With Slipped Gastric Band and Gastric Distention. Clin Gastroenterol Hepatol. 2018 Apr;16((4)):A25–6. doi: 10.1016/j.cgh.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin S, Zeikus JG. Physiological adaptations of anaerobic bacteria to low pH: metabolic control of proton motive force in Sarcina ventriculi. J Bacteriol. 1987 May;169((5)):2150–7. doi: 10.1128/jb.169.5.2150-2157.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowe SE, Pankratz HS, Zeikus JG. Influence of pH extremes on sporulation and ultrastructure of Sarcina ventriculi. J Bacteriol. 1989 Jul;171((7)):3775–81. doi: 10.1128/jb.171.7.3775-3781.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jehangir A, Rettew A, Shaikh B, Bennett K, Qureshi A, Jehangir Q. A case report of emphysematous gastritis in a diabetic patient: favorable outcome with conservative measures. J Community Hosp Intern Med Perspect. 2015 Sep;5((4)):28010. doi: 10.3402/jchimp.v5.28010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Medina G, Castillo Díaz de León R, Heredia-Salazar AC, Hernández-Salcedo DR. Gastric emphysema a spectrum of pneumatosis intestinalis: a case report and literature review. Case Rep Gastrointest Med. 2014;2014:891360. doi: 10.1155/2014/891360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karaosmanoğlu D, Oktar SO, Araç M, Erbaş G. Case report: portal and systemic venous gas in a patient after lumbar puncture. Br J Radiol. 2005 Aug;78((932)):767–9. doi: 10.1259/bjr/16733207. [DOI] [PubMed] [Google Scholar]
- 13.Liebman PR, Patten MT, Manny J, Benfield JR, Hechtman HB. Hepatic—portal venous gas in adults: etiology, pathophysiology and clinical significance. Ann Surg. 1978 Mar;187((3)):281–7. doi: 10.1097/00000658-197803000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsushima K, Won EJ, Tangel MR, Enomoto LM, Avella DM, Soybel DI. Emphysematous gastritis and gastric emphysema: similar radiographic findings, distinct clinical entities. World J Surg. 2015 Apr;39((4)):1008–17. doi: 10.1007/s00268-014-2882-7. [DOI] [PubMed] [Google Scholar]
- 15.Nemakayala DR, Rai MP, Rayamajhi S, Jafri SM. Role Of Conservative Management In Emphysematous Gastritis. BMJ Case Rep. 2018:2018. doi: 10.1136/bcr-2017-222118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yalamanchili M, Cady W. Emphysematous gastritis in a hemodialysis patient. South Med J. 2003 Jan;96((1)):84–8. doi: 10.1097/01.SMJ.0000048085.35271.75. [DOI] [PubMed] [Google Scholar]
- 17.Jung JH, Choi HJ, Yoo J, Kang SJ, Lee KY. Emphysematous gastritis associated with invasive gastric mucormycosis: a case report. J Korean Med Sci. 2007 Oct;22((5)):923–7. doi: 10.3346/jkms.2007.22.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szuchmacher M, Bedford T, Sukharamwala P, Nukala M, Parikh N, Devito P. Is surgical intervention avoidable in cases of emphysematous gastritis? A case presentation and literature review. Int J Surg Case Rep. 2013;4((5)):456–9. doi: 10.1016/j.ijscr.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


