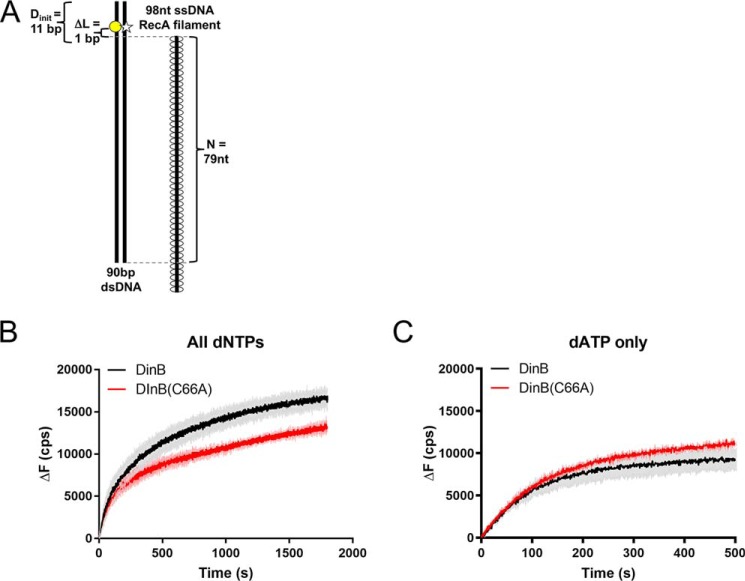
Figure 6.
DinB(C66A) can insert a single nucleotide during strand exchange as well as DinB. A, RecA-dependent strand-exchange experiments were performed similarly to Fig. 5, except fluorophores are located 1 bp away from the region of homology between the dsDNA and the ssDNA RecA filament (ΔL = 1 bp). Only a single nucleotide insertion is required to separate fluorophores and relieve quenching. B, similar to Fig. 5, experiments used all four dNTPs. Initial rates of DinB (68.48 ± 3.769 cps) and DinB(C66A) (61.86 ± 2.306) reactions were not significantly different. The DinB reaction reaches a significantly higher maximum fluorescence (17,163 ± 913 cps) than DinB(C66A) (13,859 ± 580 cps; p value < 0.01). Experiments were performed in triplicate. Mean ± S.D. (shaded region surrounding curves) is shown. C, experiments were performed as in B, except that only dATP, the nucleotide required for a single insertion, was included. The DinB reaction has a significantly lower initial rate (56.31 ± 0.8425 cps) than the DinB(C66A) reaction (58.57 ± 0.6877 cps, p value < 0.05). The maximum ΔF values reached by DinB (9756 ± 1267 cps) and DinB(C66A) (11,679 ± 180 cps) were not significantly different. Experiments were performed in triplicate. Mean ± S.D. (shaded region surrounding curves) is shown. N indicates the length of homology between dsDNA and ssDNA filament; ΔL indicates the distance between region of homology on dsDNA and fluorescent label; Dinit indicates the distance between fluorescent label and closest end of dsDNA; ΔF indicates the change in fluorescence measured in counts/s with respect to the fluorescence at 0 s. Experiments were performed in triplicate. Mean ± S.D. (shaded region surrounding curves) is shown.