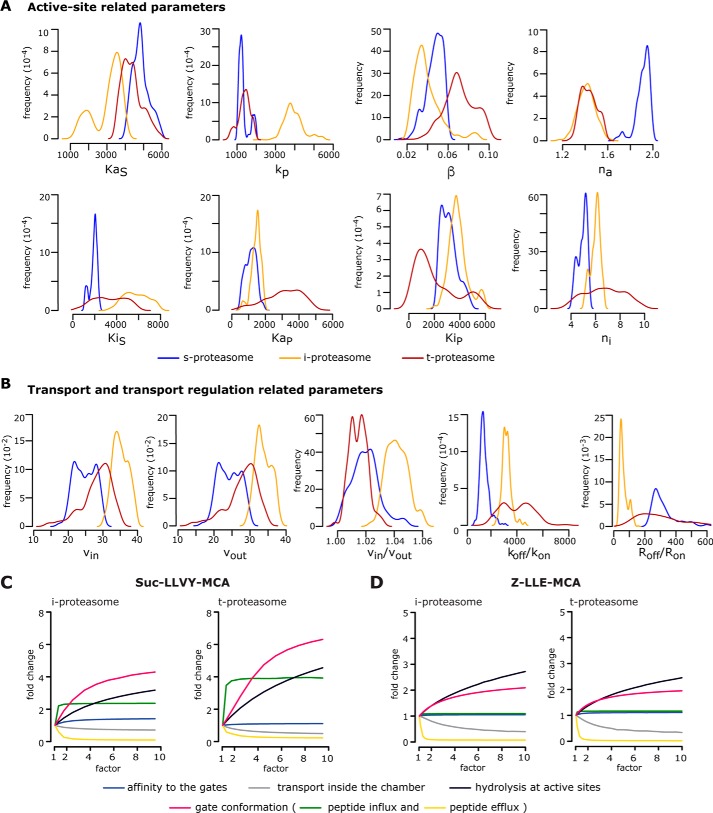
Figure 3.
Computational model parameters and rate-limiting steps of proteasome isoform dynamics. A and B, marginal posterior parameter distributions obtained by calibrating the proteasome kinetic model against experimental data (n = 3–5) derived from the degradation of the substrate Suc-LLVY-MCA by 20S s-, i-, and t-proteasomes. Parameters are grouped into active site–related parameters (A) and transport- and transport regulation–related parameters (B). Briefly, KaS and KaP are the dissociation constant of substrate (S) and product (P) to active site(s); kp is the peptide-bond hydrolysis rate at active site(s); β is the factor by which kp is multiplied upon binding to inhibitory site(s); na and ni are the Hill coefficients for binding to the active site(s) and the inhibitor site(s); KiS and KiP are the dissociation constants of substrate (S) and product (P) to inhibitor site(s); vin and vout are the peptide influx and efflux rates, and vin/vout is their ratio; koff/kon is the ratio between the dissociation and the association rates to the gate; and Roff/Ron is the ratio between the unbinding and binding rates to the enhancing regulator site(s). The meaning of all parameters is depicted in Fig. 1 and Table 1. C and D, analysis of rate-limiting steps in 20S i- and t-proteasomes. Depicted is the -fold change of product formation (y axis) upon increase (by a factor of; x axis) of a specific reaction step for the degradation of Suc-LLVY-MCA (C) and Z-LLE-MCA (D) as simulated by our computational model of the 20S proteasome dynamics. The initial substrate concentration for this analysis is 160 μm, and the -fold change is determined after 60-min reaction relative to the experimentally measured proteasome kinetics (factor = 1). The mean of 1000 in silico predictions (colored lines) is plotted over time for the degradation of the substrates with the same initial substrate concentrations as in the experiments. The rate-limiting steps are those for which the increase leads to the largest -fold change.