Figure 6.
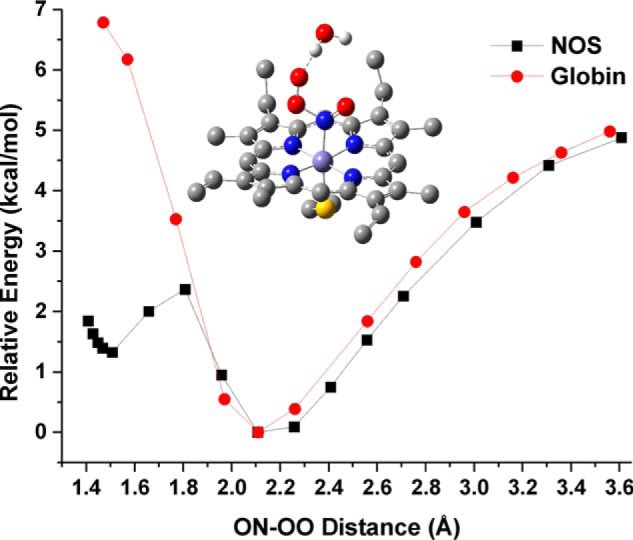
Comparison of the PES scan results for the NOS and globin active site models. Shown are the relative energies obtained from the PES scans for the reaction of the corresponding FeII-NO complexes with 3O2. Black line, the NOS active site model with the active site H2O hydrogen bond donor. Red line, the globin active site model containing the distal His (here 5-ethylimidazole) residue. Each point represents a fully optimized structure. The minimum energy calculated for the FeII-NO/O2 van der Waals complex of each model was set to 0 kcal/mol to allow for a direct comparison of the relative energies for formation of a ferric peroxynitrite intermediate. Middle, fully optimized structure of the FeIII-N(O)OO−/H2O heme-thiolate complex (ON-OO distance = 1.508 Å). The two axial hydrogen bond donors to the thiolate, the heme carboxylic acid side chains, and all protons (except those of the H2O molecule) have been removed for clarity.
