Figure 12.
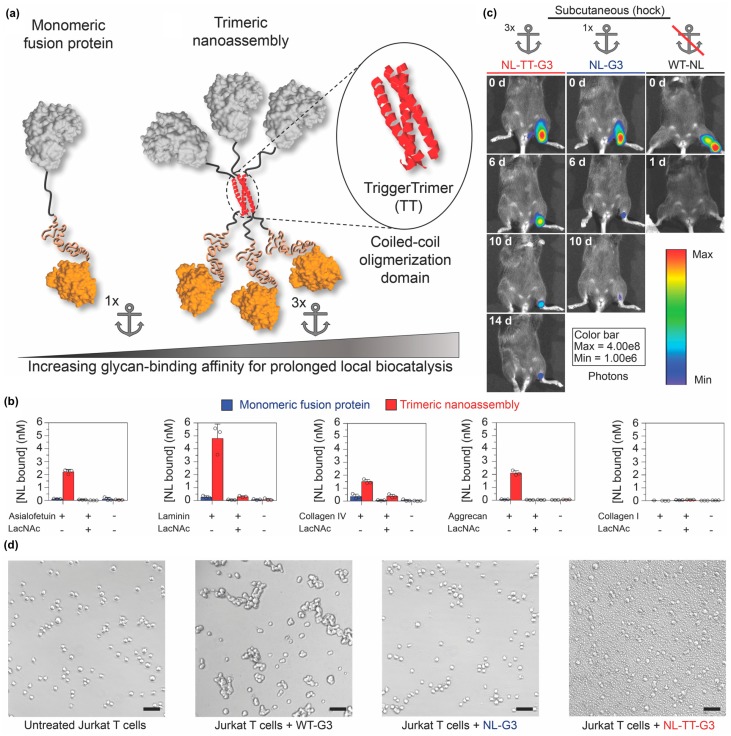
Locally anchoring enzyme nanoassemblies to tissues via Galectin-3. (a) Schematic representation of protein nanoassemblies fabricated using peptides that form an α-helical coiled-coil. (Left) A monomeric fusion protein consisting of an enzyme linked to the N-terminal domain of Galectin-3 (G3) via a flexible peptide linker. (Right) Trimeric nanoassembly formed by inserting the TriggerTrimer α-helical coiled-coil domain between the enzyme and G3 domains. The trimeric nanoassembly has higher glycan-binding affinity than the monomeric fusion protein due to multivalent avidity effects. (b) Carbohydrate-binding properties of monomeric G3 fusion proteins and trimeric nanoassemblies. (c) Bioluminescence images at various time points of mice that received trimeric nanoassemblies of NanoLuc and Galectin-3 (NL-TT-G3), a monomeric fusion protein of NanoLuc and Galectin-3 (NL-G3), or wild-type NanoLuc (WT-NL) (equivalent moles of NL) injected subcutaneously into the hock. Representative images show prolonged residence of trimeric nanoassemblies at the injection site compared with other groups. (d) Bright-field micrographs of Jurkat T cells incubated with PBS (untreated, negative control), wild-type Galectin-3 (WT-G3) (positive control), NL-G3, or NL-TT-G3 for 4 h, which demonstrated that monomeric fusion proteins and trimeric nanoassemblies lacked the activity for inducing T-cell apoptosis that is characteristic of WT-G3. Adapted with permission from reference [115] under Creative Commons Attribution 4.0 International license. Copyright 2018. Nature publishing.