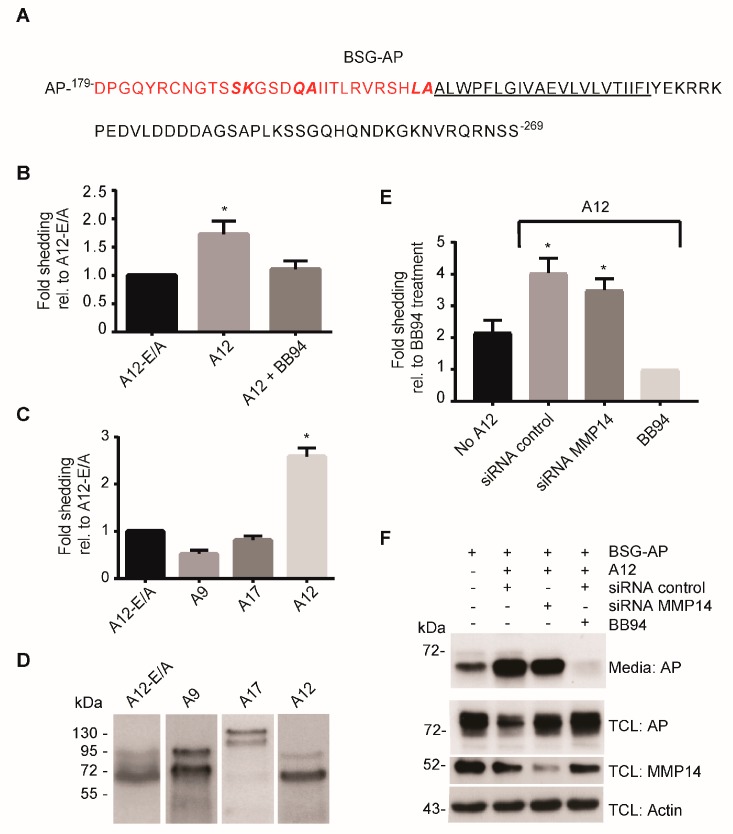
Figure 2.
ADAM12 overexpression increases ectodomain shedding of a truncated BSG reporter substrate. (A) Amino acid sequence of the reporter substrate BSG-AP, consisting of the C-terminal part of BSG fused to alkaline phosphatase (AP). The truncated extracellular part of BSG is shown in red and the transmembrane domain is underlined. (B) Fold shedding in 293-VnR cells transfected with BSG-AP together with A12 or catalytically inactive A12-E/A, and treated with or without the metalloproteinase inhibitor Batimastat (BB94). Fold shedding is calculated as AP activity in the medium divided by the total AP activity in medium and cell lysate, and normalized to A12-E/A. (C) Fold shedding in 293-VnR cells transfected with BSG-AP and A12-E/A, A12, ADAM9 (A9) or ADAM17 (A17), calculated as in (C). (D) Western blot of total lysates from cells used in (C), showing comparable expression of the different ADAMs. (E) Fold shedding in 293-VnR cells transfected with BSG-AP alone (no A12) or with BSG-AP and A12 together in cells treated with control siRNA, siRNA against matrix metalloproteinase (MMP)-14, or the inhibitor BB94 as indicated. (F) Western blot of media and total cell lysates (TCL) from cells in (E), using actin as the loading control. For all graphs, values represent means ± SEM from three independent experiments. * p < 0.05, ANOVA.