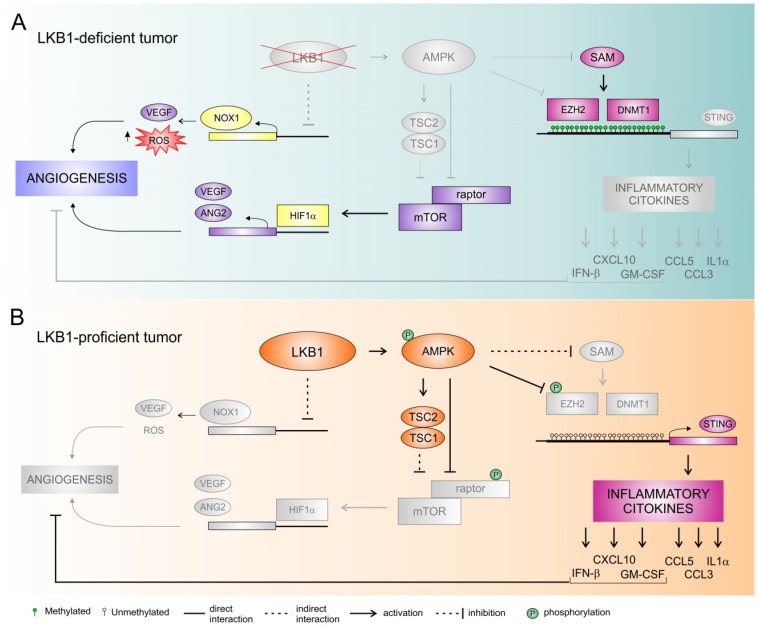
Figure 1.
LKB1-mediated regulation of tumor angiogenesis and immune escape: a working model. (A). Loss of LKB1 is associated with increased expression of NADPH oxidase 1 (NOX1) transcript. NOX1 promotes the angiogenic switch by increasing redox oxygen species (ROS) generation and expression of vascular endothelial factor (VEGF). By triggering mTOR activity, lack of AMPK activation promotes increased expression of HIF-1α and of its downstream targets, such as VEGF and angiopoetin 2 (ANG2). Moreover, loss of LKB1 promotes serine utilization and synthesis of S-adenosyl methionine (SAM), a substrate for multiple epigenetic silencing enzymes such as DNMT1 and EZH2. This results in silencing of stimulator of interferon genes (STING) expression. STING inhibition determined reduction of PD-L1 expression and downregulation of chemokines that promote T-cell recruitment, facilitating immune escape. (B). LKB1 acts as suppressor of NOX1, and, through the activation of AMPK, inhibits mTORC1, by activating the negative mTORC1 regulator TSC2 and by inhibiting the mTORC1 subunit RAPTOR. This results in reduced expression of VEGF and angiogenesis. AMPK activation inhibits methylation of the STING promoter by methyltransferases DNMT1 and EZH2. Moreover, AMPK also directly phosphorylates and inhibits EZH2. Activation of STING intracellular phosphorylation cascade led to the release of the immune inflammatory cytokines such as IFNβ, CXCL10, CCL5, GM-CSF, CCL3, and IL1α, which leads to increased antitumor innate immunity signals and higher PD-L1 expression. Moreover, IFNβ, CXCL10, and GM-CSF may also contribute to inhibition of tumor angiogenesis.