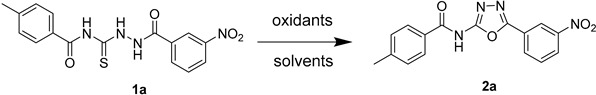
Table 1.
Optimization of cyclization of the thiosemicarbazide.
| Entry | Oxidant | Solvent | Temp. (°C) | Yield (%) |
|---|---|---|---|---|
| 1 | K2S2O8 | Water | 100 | N.O. a,c |
| 2 | (NH4)2S2O8 | Water | 100 | N.O. a,c |
| 3 | IBX | Water | 100 | <30 a |
| 4 | Oxone | Water | 100 | <40 a |
| 5 | KIO3 | Water | 100 | 53 b |
| 6 | KIO3 | Water | 80 | 83 b |
| 7 | KIO3 | Water | 40 | 46 b |
| 8 | KIO3 | Water | 60 | 90 b |
| 9 | KIO3 | DCM | 60 | 5 a |
| 10 | KIO3 | Acetone | 60 | N.O. a,c |
All the reaction were carried out on a 0.4 mmol scale with 1.5 equiv. oxidants within 2 h; a the reaction conversions were monitored by LC-MS; b Isolated yield; c N.O.: No observation.
