Figure 1.
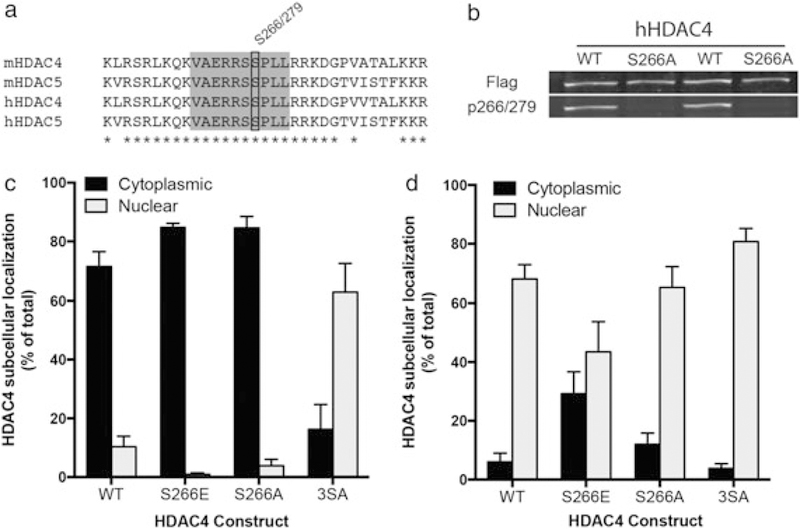
HDAC4 S266 drives cytoplasmic localization when phosphorylated. (a) Residue conservation comparison between mouse and human HDAC4 and HDAC5 near the S266/S279 residue (black box). Gray box encompasses the epitope used for antibody production; asterisks indicate 100 percent identity sites. (b) HDAC4 WT or HDAC S266A overexpressed in HEK293 cells show equivalent total HDAC expression (top panel) but the absence of p266 signal (bottom panel) in the alanine mutant condition. (c) Phosphomimetic mutation of S266 (S266E) promotes HDAC4 cytoplasmic accumulation. Mutation of critical serine residues to alanine (S246A/S467A/S632A) shifts HDAC4 subcellular localization to the nucleus. (d) Under conditions where nuclear export is blocked (3 hour of 10 ng/ml leptomycin B treatment), phosphomimetic mutation of S266 (S266E) causes enhanced cytoplasmic localization, indicating reduced nuclear import. In (c) and (d), transfected neurons were identified by GFP expression (driven by a second promoter), and subcellular localization was determined by Flag signal (C-terminal tag on all HDAC4 constructs)
