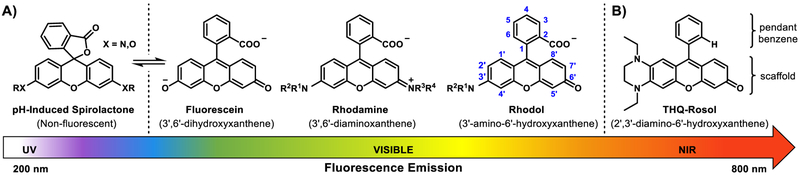
Figure 1.
Progressive exocyclic heteroatom modifications to the xanthene core structure of topologically-equivalent molecular fluorescent dyes that paved the way for the development of the first NIR fluorescent rosol dye, THQ-Rosol. A) General molecular platform of fluorescein-, rhodamine-, and rhodol-based fluorescent dyes, all of which consist of a charged non-NIR fluorescent xanthene core-based scaffold having a pendant benzene moiety with a charged 2-carboxylate group (-COO-) that promotes pH-induced spirolactonization and affords their scaffolds a pH-sensitive fluorescence response at more elevated pH levels than it otherwise demonstrates when without it.18–20 B) Molecular platform of rationally-designed THQ-Rosol, which consists of i) an uncharged NIR fluorescent scaffold that evolves from the non-NIR scaffold that also underlies the rhodol molecular platform and ii) a pendant benzene moiety without a charged 2-carboxylate group to afford an inherently uncharged molecular platform, preclude pH-induced spirolactonization, and afford the scaffold a steady maximal NIR fluorescence response at more elevated pH levels than it would otherwise demonstrate when a charged 2-carboxylate group is present.