Table 1.
Calculated, Modeled, and Measured Properties of Non-NIR Fluorescent Rosol Dyes and THQ-Rosol.
| Compound | 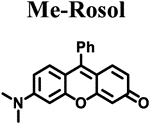 |
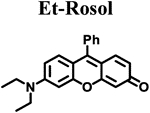 |
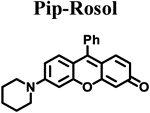 |
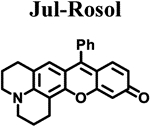 |
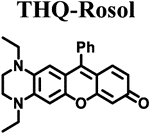 |
|---|---|---|---|---|---|
| ELUMO (hartrees)a | −0.07500 | −0.07520 | −0.07795 | −0.07281 | −0.06856 |
| EHOMO (hartrees)a | −0.18814 | −0.18811 | −0.19086 | −0.18333 | −0.17245 |
| ΔE (hartrees) | 0.11314 | 0.11291 | 0.11291 | 0.11052 | 0.10389 |
| Torsion angle N-C2’ (θ)b | - | - | - | - | 12.21° |
| Torsion angle N-C3’ (θ)b | 18.57° | 14.86° | 12.01° | 7.45° | 10.34° |
| λabs (nm)c | 520 | 524 | 525 | 540 | 550 |
| λem (nm)d | 557 | 558 | 563 | 574 | 710 |
| Stokes shift (nm) | 37 | 34 | 38 | 34 | 160 |
| Photostability (I/I0)e | 0.99 | 0.98 | 0.97 | 0.98 | 0.99 |
| ε (M−1 cm−1) | 46,136 | 42,629 | 42,460 | 30,420 | 29,382 |
| Φflf | 0.182 ± 0.007 | 0.098 ± 0.001 | 0.048 ± 0.001 | 0.414 ± 0.025 | 0.029 ± 0.001 |
| Brightness (M−1cm−1) | 8.4 | 4.2 | 2.0 | 13 | 0.85 |
| pKag | 4.84 | 4.97 | 4.95 | 5.76 | 5.85 |
DFT calculations were performed on the truncated scaffolds of each modeled fluorescent rosol dye to obtain their respective ELUMO and EHOMO values.
The modeled torsion angle is the offset from planarity (in degrees) of the alkyl substituent that is appended to the C2’- or C3’-nitrogen atom in relation to their respective xanthene core-based scaffold.
Measured maximum absorption wavelength of the fluorescent rosol dye (20 μM) in aqueous buffer (50 mM phosphate, 150 mM NaCl, pH 7.4).
Measured maximum emission wavelength of the fluorescent rosol dye (20 μM) upon exciting it at its respective λabs in aqueous buffer (50 mM phosphate, 150 mM NaCl, pH 7.4).
I0 is the measured initial fluorescence intensity of the fluorescent rosol dye and I is its measured fluorescence intensity after 30 min of continuous photoirradiation.
Φfl is the measured fluorescence quantum yield.
pKa values were determined using 20 μM of the fluorescent rosol dye in aqueous buffer (50 mM phosphate, 150 mM NaCl, pH 7.4). Error in Φfl and pKa values are ±5% based on triplicate measurements.
