Abstract
This letter to the editor describes myocarditis screening among patients undergoing combination immune checkpoint inhibitor therapy, in light of the consensus document from the Checkpoint Inhibitor Safety Working Group.
The consensus document from the Checkpoint Inhibitor Safety Working Group identified monitoring strategies as a key information gap in the realm of immune checkpoint inhibitor (ICI)‐associated myocarditis [1]. Given its potentially fulminant course, experts have proposed screening algorithms using serial troponin (Tn) and/or electrocardiogram (ECG) for early detection of subclinical disease and prompt initiation of therapy to potentially mitigate cardiac morbidity and mortality [2], [3], [4].
We evaluated screening troponin I (TnI) and ECG in 76 asymptomatic patients with advanced melanoma undergoing combination ICI therapy with ipilimumab and nivolumab. Baseline and weekly TnI/ECG were obtained until the second dose, corresponding to the typical timing of onset, for a maximum of four samples. Outcomes of interest were myocarditis, major adverse cardiac events (MACE), and all‐cause mortality.
The median age was 65 years (interquartile range 57–70), with 45 (59.2%) male patients. The prevalence of coronary artery disease, hypertension, hypercholesterolemia, and diabetes mellitus was 10.5% (n = 8), 40.8% (n = 31), 34.2% (n = 26), and 13.2% (n = 10), respectively. Thirty‐one (40.3%) patients had previously received ICI therapy, one (1.3%) with doxorubicin and three (3.9%) mediastinal radiation. Over half (n = 49, 64.5%) developed noncardiac immune‐related adverse events, of which 37 received corticosteroids at some point during the course of combination ICI therapy.
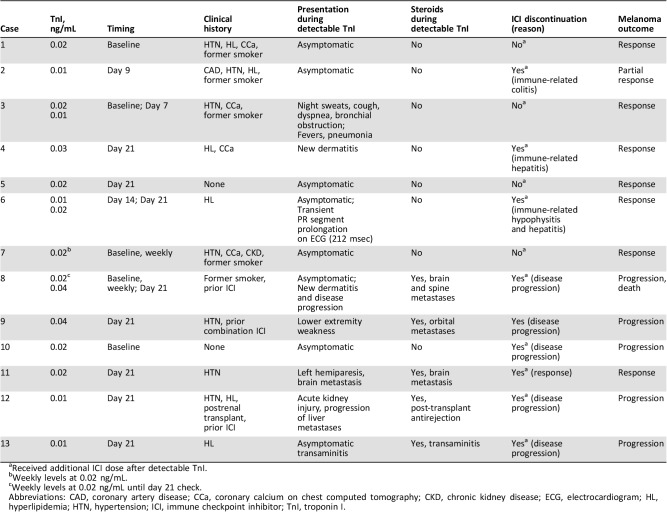
Over a median of 198 days, none of the 76 patients developed clinical or subclinical myocarditis or MACE. All deaths (n = 17, 22.4%) were cancer related. Additionally, none had elevated TnI levels (>0.64 ng/mL) or ECG abnormalities. Minimally detectable nondiagnostic TnI levels (≥0.01 ng/mL and <0.06 ng/mL) were seen in 13 (17%) patients only after adoption of a higher sensitivity assay (Table 1). At the time of detectable TnI, all remained asymptomatic and hemodynamically stable, and complementary testing not limited to serial TnI/ECGs did not identify any obvious acute cardiac and/or systemic pathology. At follow‐up, 11 patients had undetectable TnI, and two had nontrending levels. There was no association between detectable TnI and all‐cause mortality. Twelve of 13 received further ICIs without cardiac events, and one discontinued ICI therapy because of disease progression.
Table 1. Detectable troponin I case description.
Received additional ICI dose after detectable TnI.
Weekly levels at 0.02 ng/mL.
Weekly levels at 0.02 ng/mL until day 21 check.
Abbreviations: CAD, coronary artery disease; CCa, coronary calcium on chest computed tomography; CKD, chronic kidney disease; ECG, electrocardiogram; HL, hyperlipidemia; HTN, hypertension; ICI, immune checkpoint inhibitor; TnI, troponin I.
This is the first report on myocarditis screening among patients undergoing combination ICI therapy. Despite inclusion of a higher‐risk cohort, none of our patients developed myocarditis. We also demonstrated that ICIs can be safely continued in asymptomatic, hemodynamically stable patients with minimally detectable TnI levels after careful consideration of the clinical context. Likewise, the study by Sarocchi et al. of 59 patients undergoing serial TnI checks during nivolumab monotherapy had one patient with presumed subclinical myocarditis who tolerated continued ICI therapy without cardiac events [5]. These minor nondiagnostic TnI levels could cloud diagnostic judgment, initiate unnecessary downstream testing and treatments, and interfere with the course of potentially life‐saving cancer treatment. As more institutions adopt higher sensitivity assays, there is a trend toward increased Tn detection at a loss of discriminatory capacity, without improvement in short‐term cardiovascular outcomes [6], [7].
Our study is reflective of the low prevalence of ICI‐associated myocarditis and, therefore, low screening yield. However, it is possible that a larger study with improved risk stratification and patient selection could prove some utility for TnI/ECG screening.
Acknowledgments
The study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center (IRB MED18‐009). Requirement for written informed consent was waived. This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. The authors acknowledge the Ludwig Institute for Cancer Research and Swim Across America Laboratory, and Parker Institute for Cancer Immunotherapy (all at Memorial Sloan Kettering Cancer Center in New York, NY).
Disclosures
Michael A. Postow: Bristol‐Myers Squibb, Merck, Array BioPharma, Novartis, Incyte, NewLink Genetics, Aduro (C/A), Bristol‐Myers Squibb, Merck (H), RGenix, Infinity, Bristol‐Myers Squibb, Merck, Array BioPharma, Novartis, AstraZeneca (other—institutional support); Margaret K. Callahan: Merck, Moderna, AstraZeneca, InCyte (C/A), Bristol‐Myers Squibb (RF, E—family member); Paul B. Chapman: Pfizer (RF), Merck, Immunocore, Cell Medica, Takeda Millennium (C/A); Alexander N. Shoushtari: Bristol‐Myers Squibb, Castle Biosciences, Immunocore (C/A), AstraZeneca, Bristol‐Myers Squibb, Immunocore (RF); Tomas G. Neilan: Parexel, Intrinsic Imaging (C/A); Michael G. Fradley: Novartis (C/A); Jedd D. Wolchok: Adaptive Biotech, Advaxis, Amgen, Apricity, Array BioPharma, Ascentage Pharma, Beigene, Bristol‐Myers Squibb, Celgene, Chugai, Elucida, Eli Lilly & Co., F Star, Genentech, Imvaq, Janssen, MedImmune, Merck, Neon Therapeutics, Ono, Polaris Pharma, Polynoma, Psioxus, Puretech, Recepta, Trienza, Sellas Life Sciences, Surface Oncology, Syndax (C/A), Bristol‐Myers Squibb, MedImmune, Merck Pharmaceuticals, Genentech (RF), Potenza Therapeutics, Tizona Pharmaceuticals, Adaptive Biotechnologies, Elucida, Imvaq, Beigene, Trieza (OI), Linneaus (IP). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Neilan TG, Rothenberg ML, Amiri‐Kordestani L et al. Myocarditis associated with immune checkpoint inhibitors: An expert consensus on data gaps and a call to action. The Oncologist 2018;23:874–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahmood SS, Fradley MG, Cohen JV et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang DY, Okoye GD, Neilan TG et al. Cardiovascular toxicities associated with cancer immunotherapies. Curr Cardiol Rep 2017;19:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarocchi M, Grossi F, Arboscello E et al. Serial troponin for early detection of nivolumab cardiotoxicity in advanced non‐small cell lung cancer patients. The Oncologist 2018;23:936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melki D, Lugnegard J, Alfredsson J et al. Implications of introducing high‐sensitivity cardiac troponin T into clinical practice: Data from the SWEDEHEART registry. J Am Coll Cardiol 2015;65:1655–1664. [DOI] [PubMed] [Google Scholar]
- 7.Love SA, Sandoval Y, Smith SW et al. Incidence of undetectable, measurable, and increased cardiac troponin I concentrations above the 99th percentile using a high‐sensitivity vs a contemporary assay in patients presenting to the emergency department. Clin Chem 2016;62:1115–1119. [DOI] [PubMed] [Google Scholar]