Figure 6.
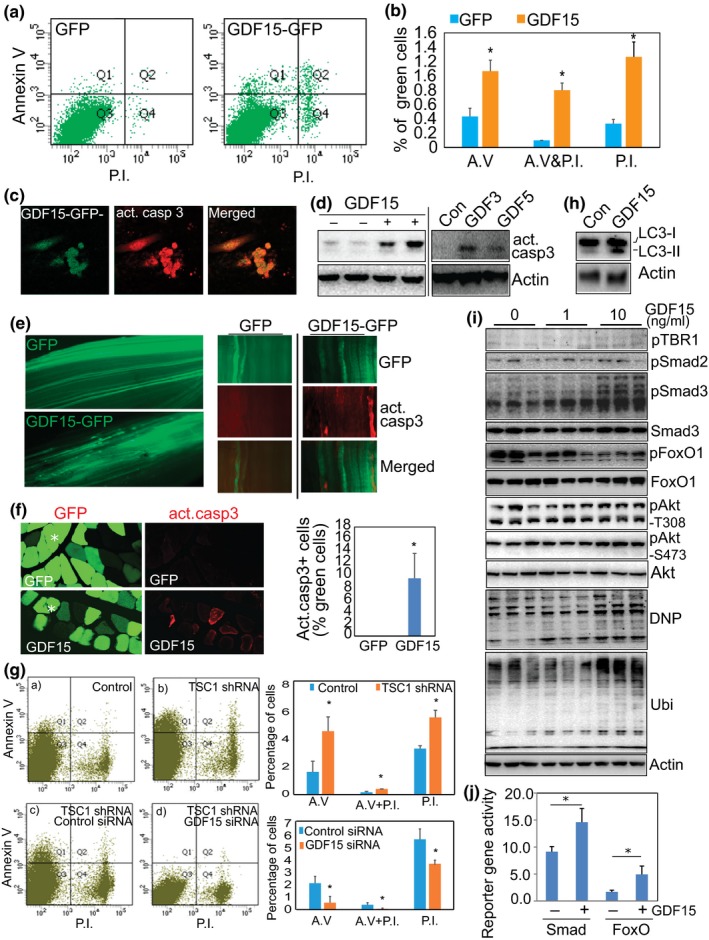
GDF is sufficient to induce apoptosis in muscle cells and is required for mTORC1‐induced apoptosis through regulation of Smad3, FoxO1, and oxidative stress (a) GDF15 induces apoptotic changes in cultured cells. C2C12 cells were transfected with GDF15‐GFP and control GFP plasmids. One day later, cells were harvested, stained with Annexin V and Propidium Iodide (PI), and analyzed by flow cytometry. Shown is the distribution of GFP+ and GDF15‐GFP+ cells. (b) Quantitative data of the distribution of the positively stained cells in the different quadrants. n = 3, *p < 0.05. (c) GDF15‐transfected cells show positive staining of cleaved caspase 3. Immunostaining was performed with anticleaved caspase 3 (red), 3 days after transfection of GDF15‐GFP (green) into C2C12 cells. (d) GDF increases the protein level of activated/cleaved caspase 3. Recombinant GDF3, 5, and 15 (10 ng/ml) was used to treat differentiated C2C12 myotubes for 4 days, followed by detection of cleaved caspase 3 by Western blot analysis. (e and f) GDF15 activates caspase 3 in vivo. TA muscle was electroporated with GDF15‐GFP or control GFP plasmids (20 μg/injection). Fourteen days later, TA muscles were harvested and fixed with paraformaldehyde for whole‐mount staining (e) or cross‐sectional staining (f) with anticleaved caspase 3. Note that some GDF15+ fibers stain positively for cleaved/activated caspase 3 (red). (g) GDF15 is required for the development of apoptosis induced by activated mTORC1. A stable C2C12 cell line that overexpresses TSC1 shRNA was transfected with control and GDF15 siRNAs (each at 20 nM, Santa Cruz Biotechnology) to silence GDF15. Flow cytometry was performed 4 days after transfection. Activation of mTORC1 induces apoptotic markers (top panel), but the induced apoptosis was significantly suppressed by transfected GDF15 siRNA (lower panel). n = 3, *p < 0.05. (h) GDF15 increases the levels of autophagic marker, LC3, revealed by Western blot analysis. Increased protein levels of both LC3‐I and LC3‐II, 3 days after transfection of GDF15 into C2C12 cells. (i) GDF15 induces protein oxidation (DNP), polyubiquitination (Ubi), and activation of Smad3 and FoxO1. C2C12 myotubes were treated with recombinant GDF15 protein at 1 and 10 ng/ml for 48 hr. Western blot analysis is shown. Note that GDF15 phosphorylates Smad3 and dephosphorylates FoxO1. (j) GDF15 increases the activity of FoxO's and Smad's reporter gene. GDF15‐expressing plasmid was cotransfected into C2C12 cells, together with a FoxO1 reporter gene 6DBE, or a Smad3 reporter gene 3TP‐Lux, as well as a nonreporter control pCS2‐beta‐Gal, respectively. Luciferase (reporter) and beta‐Gal (nonreporter control) activities were measured 3 days later, and luciferase activity was normalized to beta‐Gal activity
