Figure 7.
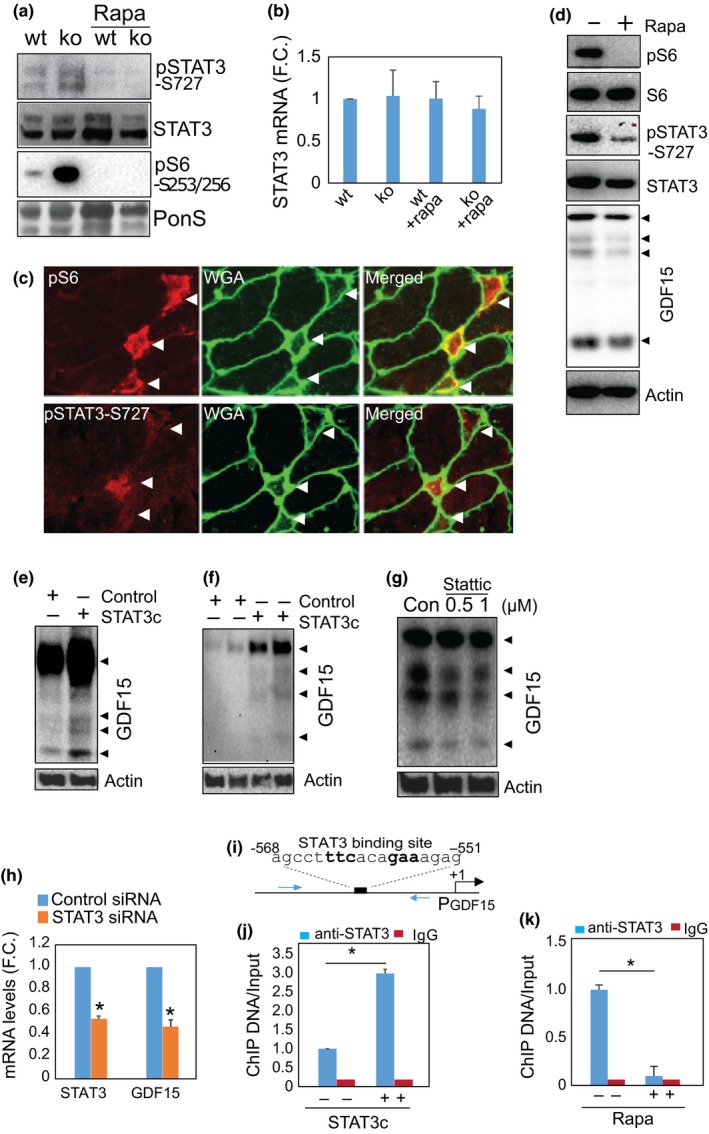
mTORC1‐activated STAT3 transcriptionally upregulates GDF15. (a) mTORC1 activity regulates the phosphorylation of STAT3 in mTORC1‐activated muscle. Western blot analysis was performed with TA muscle lysate. n = 3, representative picture was shown. Note, mTORC1‐induced STAT3 phosphorylation is suppressed by rapamycin treatment. (b) mTORC1 activity does not regulate STAT3 mRNA levels. Quantitative PCR was performed to measure mRNA levels in gastrocnemius muscle. n = 4 per group. (c) STAT3 is phosphorylated in pS6+ muscle fibers in aged muscle. Immunostaining was performed to detect phosphorylated S6 and STAT3 (S727) with specific antibodies. Arrowheads indicate positively stained muscle fibers. (d) Inhibition of mTORC1 activity suppresses STAT3 phosphorylation and the expression of GDF15 in cultured myotubes. Rapamycin, 2 nM, for 24 hr. Experiments were repeated three times, and representative images were shown. (e and f) Overexpression of STAT3 increases the expression of GDF15 in vitro and in vivo. Constitutive active STAT (STAT3c) was transfected into C2C12 cells (e) or electroporated into TA muscle (f). GDF15 levels were examined by Western blot analysis. (g) Inhibition of STAT3 activity suppresses the expression of GDF15. STAT3 inhibitor, Stattic, was used to treat cultured myotubes for 24 hr, and GDF15 levels were examined by Western blot analysis. Experiments were repeated three times and representative image was shown. (h) Silencing STAT3 reduces GDF15 expression. siRNA against STAT3 was transfected into C2C12 cells and RNA was harvested 4 days later for quantitative PCR. n = 4, *p < 0.05. (i, j, and k) STAT3 binds to GDF15 promoter. Consensus sequence of STAT3 binding is present in the promoter sequence of GDF15 (i). ChIP assay was performed with STAT3 transfected C2C12 cells (j) and rapamycin‐treated C2C12 myotubes (k). n = 4 each, *p < 0.05. Increased STAT3 level increases STAT3's DNA binding, whereas rapamycin treatment reduces STAT3 binding to the GDF promoter
