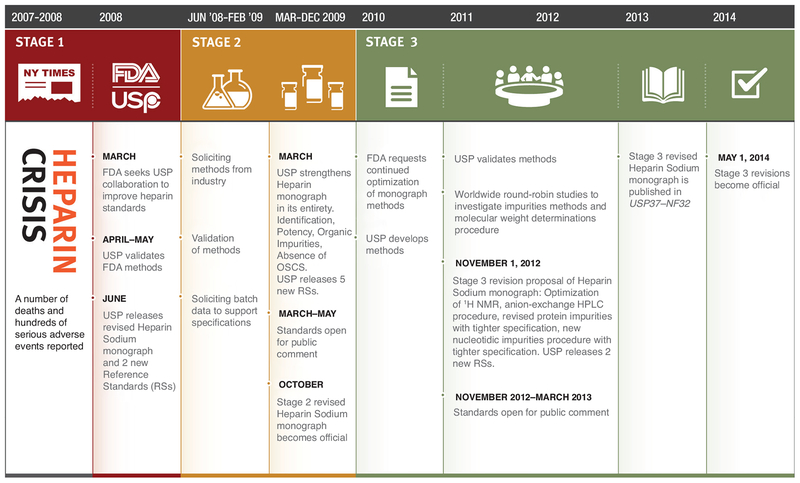
Figure 1.
Heparin crisis and resolution timeline. After the contamination crisis, the USP undertook and concluded a three-stage set of revisions to the Heparin Sodium monograph. Stage 1 involved the initial addition of identity tests and introduction of reference standards to prevent contaminated heparins from entering the US market. Stage 2 strengthened the entire monograph by including specific potency testing, additional tests and tightening existing limits for impurities. Stage 3 focused on further tightening limits for impurities and controlling heparin’s polydispersity by molecular weight analysis.